Abstract
Deadwood is a key component of forest ecosystems, but there is limited information on how it influences forest soils. Moreover, studies on the effect of thinning-derived deadwood logs on forest soil properties are lacking. This study aimed to investigate the impact of thinning-derived deadwood logs on the soil chemical and microbial properties of a managed spruce forest on a loamy sand Podzol in Bavaria, Germany, after about 15 years. Deadwood increased the soil organic carbon contents by 59% and 56% at 0–4 cm and 8–12 cm depths, respectively. Under deadwood, the soil dissolved organic carbon and carbon to nitrogen ratio increased by 66% and 15% at 0–4 cm depth and by 55% and 28% at 8–12 cm depth, respectively. Deadwood also induced 71% and 92% higher microbial biomass carbon, 106% and 125% higher microbial biomass nitrogen, and 136% and 44% higher β-glucosidase activity in the soil at 0–4 cm and 8–12 cm depths, respectively. Many of the measured variables significantly correlated with soil organic carbon suggesting that deadwood modified the soil biochemical processes by altering soil carbon storage. Our results indicate the potential of thinned spruce deadwood logs to sequester carbon and improve the fertility of Podzol soils. This could be associated with the slow decay rate of spruce deadwood logs and low biological activity of Podzols that promote the accumulation of soil carbon. We propose that leaving thinning-derived deadwood on the forest floor can support soil and forest sustainability as well as carbon sequestration.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Forests constitute about 30% (4–5 billion hectares) of the earth’s terrestrial area, providing many essential ecosystem services (Keenan et al. 2015; Bastin et al. 2017). Globally, forests contain approximately 860 Pg carbon (C), which equates to nearly 50% of the total organic C in terrestrial ecosystems (IPCC 2007; Wei et al. 2014). This highlights the remarkable capacity of forest soils to act as a sink and source of carbon dioxide (CO2) that regulate global CO2 dynamics and C sequestration (Bonan 2008; Neruda et al. 2010). Total soil C stocks compose about 60% of the ecosystem C stock in temperate forests (Pan et al. 2011), demonstrating its importance for the ecosystem balance and functioning in temperate regions like Europe. For instance, maintaining and enhancing forest soil C stocks support soil C cycling, fertility, water availability, biodiversity, and forest productivity (Page-Dumroese and Jurgensen 2006; Mayer et al. 2020).
Alterations to inputs of soil organic matter (SOM) and the loss of C affect the soil C balance. Such modifications can be natural (e.g., wildfire and pests) or anthropogenic (e.g., land use and management) (Nave et al. 2010; Zhang et al. 2015; James and Harrison 2016). Forest thinning is a management practice that selectively removes trees to increase the availability of resources to the remaining trees to improve their growth and productivity. Thinning regulates forest structure, reduces the risk of wildfires, enhances timber production, and increases forest resilience to environmental disturbances (Makinen and Isomaki 2004; Sohn et al. 2016; Wang et al. 2017a, b). Despite these benefits, thinning decreases C stocks of forest soils due to reduced litter and root exudate inputs and increased rates of SOM mineralization (Vesterdal et al. 1995; Jandl et al. 2007; Laganiere et al. 2010; Moreno-Fernández et al. 2015; Zhang et al. 2018; Mushinski et al. 2019). Specifically, thinning increases the risk of C and nutrient limitations in coarse-textured soils of low fertility (e.g., Podzol) compared to fine-textured fertile soils (Morris 1997; Page-Dumroese et al. 2010).
Thinning leads to reductions in deadwood (e.g., tree logs and branches) remaining on the forest floor, because thinned trees are removed for biofuel or industrial purposes (Page-Dumroese et al. 2010; Powers et al. 2011; Zhou et al. 2013). This can further increase the scarcity of deadwood in managed Central European forests compared to unmanaged forests (Ministerial Conference on the Protection of Forests in Europe, 2015). Deadwood, particularly as logs, is an important habitat and source of nutrients for many soil- and wood-inhabiting organisms, such as insects and fungi (Grove 2002; Perreault et al. 2020). Deadwood-derived C can enter soil as dissolved organic matter through infiltrating water (i.e., by precipitation) or as fragments through bioturbation (i.e., by insects and worms) influencing various soil properties, such as organic matter, pH, microbial biomass, and enzyme activity (Krzyszowska-Waitkus et al. 2006; Kappes et al. 2007; Allison and Martiny 2008; Ma et al. 2014). Studies show that organic C and nutrients are higher in soils under deadwood (Goldin and Hutchinson 2013; Bade et al. 2015; Stutz et al. 2017), while others demonstrate no or even negative changes in the concentration of soil C and nutrients under deadwood (Spears et al. 2003; Kahl et al. 2012; Zalamea et al. 2016). Retaining deadwood on soil is seldom incorporated into forest management plans due to the inconclusiveness of past research (Magnússon et al. 2016; Gonzalez-Polo et al. 2013; Stutz and Lang 2017) and limited understanding, especially in European temperate forest ecosystems (Lombardi et al. 2013).
No previous studies evaluated the impact of thinning-derived deadwood logs on forest soil properties. Former studies were concerned with deadwood from trees that collapsed by natural phenomena or manually placed deadwood of different decay classes (e.g., Chang et al. 2017; Peršoh and Borken 2017; Stutz et al. 2017). An innovative and applied forest management practice can be the retention of deadwood derived from thinning operations on forest soils. This study aimed to investigate the impact of deadwood from forest thinning on the soil chemical and microbial properties of an acidic Podzol with loamy sand texture after prolonged time (~ 15 years). The main hypothesis of this study was that thinning-derived deadwood significantly increases the SOC content, microbial biomass, and enzyme activity.
Materials and methods
Study site and field design
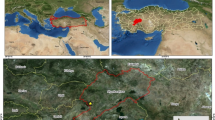
The study site is a managed 55 years old spruce (Picea abies) forest located at Kulmbach, Bavaria, Germany (50°6′30″ N and 11°29′12″ E) with an altitude of 360 m above sea level and a north-facing slope of 30° (Fig. 1A). The average annual temperature and cumulative precipitation are 9.1 °C and 957 mm, respectively. The soil is classified as Podzol (Federal Institute for Geosciences and Natural Resources, Germany) with a loamy sand texture (US soil taxonomy). The forest had been thinned in 2006 and downed spruce deadwood logs had been randomly distributed throughout the forest floor. Four deadwood logs (n = 4) of similar length (~ 80 cm), diameter (~ 60 cm), and decay class that were in complete contact with the forest floor were selected. We selected deadwood logs positioned perpendicular to the slope to avoid any redundant accumulation of water and litter on one side (Fig. 1B). After removing the organic layer, intact soil samples were taken from the mineral horizon under the center of each deadwood log (deadwood) and at a distance of 3 m from the deadwood log (control) at 0–4 cm and 8–12 cm depths. The control samples were taken at a distance of 3 m from the deadwood logs to avoid any influence of deadwood and its derivates. Samples were taken from the two different depths to represent the impact by direct contact (0–4 cm) and by translocation of substrates (8–12 cm). Soil sampling was performed in April 2021 using 100 cm3 (25 cm2 × 4 cm) metal rings. Before analysis, soil samples were sieved (< 2 mm) and stored in polyethylene bags at 4 °C.
White-rot fungi were visually identified on all selected deadwood logs (Fig. 1C). The decay class of the deadwood logs was determined by the “pocket-knife” method (Lachat et al. 2014). The selected deadwood logs had a moderate decay class, meaning that a pocket knife easily penetrated with fibers, but not across. Moss and fern were the dominant vegetation on the forest floor.
Laboratory analyses
The soil pH and electrical conductivity (EC) were determined at a 1/2.5 (w/v) soil-to-water ratio. The soil total C and total nitrogen (N) were measured by dry combustion using an Elemental Analyzer (Euro EA –CN, Eurovector, Pavia, Italy). Due to the absence of carbonates and low soil pH, the total C was considered soil organic carbon (SOC). The soil microbial biomass carbon (MBC) and microbial biomass nitrogen (MBN) were determined by the fumigation-extraction method (Brookes et al. 1985; Wu et al. 1990). Briefly, two portions of 15 g soil were taken from the soil samples. One portion was fumigated for 24 h at 25 °C with 50 ml ethanol-free chloroform (CHCl3). Then, the fumigated and non-fumigated soil portions were extracted with 60 mL 0.5 M K2SO4 (potassium sulfate), filtered, and frozen. After thawing, C and N in the frozen extracts were measured by the multi N/C 2100 (Analytik Jena, Germany) automated analyzer. MBC was calculated as ExC/0.45, where ExC = (extractable C from fumigated samples)–(extractable C from non-fumigated samples) (Wu et al. 1990). MBN was ExN/0.54, where ExN = (extractable N from fumigated samples) – (extractable N from non-fumigated samples) (Brookes et al. 1985). K2SO4 extractable C from fumigated samples was taken as the index for dissolved organic carbon (DOC) (Wu et al. 1990; Nazari et al. 2020).
The kinetics of β-glucosidase, cellobiosidase, and chitinase enzymes were determined using β-D-glucoside, β-D-cellobioside, and N-acetyl-β-D-xylopyranoside fluorogenic substrates, respectively (Sigma Aldrich, Germany) following (Marx et al. 2005). Briefly, 1 g soil was suspended in 50 mL distilled water, of which 50 μL suspension was pipetted in a 96-well microplate (Thermo Fisher, Denmark). Then, 50 μL MES buffer (pH 6.5) and 100 μL respective substrate solution were subsequently added to each well. The activity of each enzyme was measured at 3-time points of 30, 60, and 120 min using CLARIOstar plus (BMG LABTECH, Germany) at the excitation and emission wavelengths of 355 nm and 460 nm, respectively. Enzyme activities (Vmax) were denoted as released MUF (4 methylumbelliferone) in nmol per g dry soil per hour (nmol MUF g−1 soil h−1) (Awad et al. 2012) and affinity constant for each enzyme (Km) expressed in μmol substrate per g dry soil (μmol g−1 soil). Low Km indicates high enzyme efficiency and vice versa. To ensure the saturation concentrations of fluorogenic substrates, enzyme activities over a range of substrate concentrations from low to high (0, 10, 20, 30, 40, 50, 100, 200 μmol g−1 soil) were determined. Besides, the linear increase in fluorescence over time during the assay was properly checked and data, which was obtained after 2 h, was used for further calculation (German et al. 2011). MUF concentrations of 0, 10, 20, 30, 40, 50, 100, and 200 μmol were prepared to calibrate the measurement in the same plate as well.
The Michaelis–Menten equation was used to determine the parameters of the enzyme activity (V):
where Vmax is the maximum enzyme activity; Km represents the half-saturation constant, or the substrate concentration at which the reaction rate equals Vmax/2; and S is the substrate concentration at the active site of the enzyme. Both Vmax and Km parameters were obtained from the Michaelis–Menten Eq. (1) using the nonlinear regression routine of Sigmaplot.
Statistical analyses
All data were analyzed by IBM SPSS Statistics for Windows, version 25 (IBM Corp., Armonk, NY, United States). Levene’s test and the Shapiro–Wilk test were used to check for homogeneity of variances and normality of the data, respectively. The data violating these assumptions were properly transformed. Significance of effects of deadwood and soil depth were tested by two-factorial analysis of variance (ANOVA) at the significance level α = 0.05. Pearson’s correlation test was used to detect significant relationships between the measured soil variables at α = 0.01. The bar plots were designed using SigmaPlot 14.0 (Systat, San José, CA, United States).
Results
Soil chemical properties
The soil pH and EC ranged from 4.19–4.31 to 24.03–43.23 µS cm−1, respectively, and were not significantly affected by deadwood at any depth (Table 1). Deadwood considerably increased the SOC by 59% and 56% at 0–4 cm and 8–12 cm depths, respectively, the enhancements were however not statistically significant (Fig. 2A). The soil total N was not significantly influenced by deadwood at any depth (Fig. 2B). The SOC and total N contents were significantly lower at 8–12 cm than at 0–4 cm depth. Under deadwood, the ratio of SOC to total N (C:N) increased significantly by 15% and 28% at 0–4 cm and 8–12 cm depths, respectively, and decreased by depth (Fig. 2C). Deadwood significantly increased the soil extractable C by 66% and 55% at 0–4 cm and at 8–12 cm depths, respectively (Fig. 3A). However, the contribution of the extractable C fraction to SOC content was significantly affected only by depth rather than by deadwood, being 30% greater at 8–12 cm depth than at 0–4 cm depth (Fig. 2D).
Effect of thinning-derived deadwood on the soil organic carbon (SOC) content (A), total nitrogen (Total N) content (B), carbon to nitrogen ratio (C:N) (C), and extractable carbon fraction in soil organic carbon expressed in % (D) of the studied temperate spruce forest in Kulmbach, Bavaria, Germany (factorial ANOVA test at α = 0.05, n = 4). P-values ≤ 0.05 indicate a significant effect. Error bars show the standard error of the mean
Effect of thinning-derived deadwood on the soil extractable carbon (DOC) (A), β-glucosidase activity (Vmax) (B), chitinase activity (C), and cellobiosidase activity (D) of the studied temperate spruce forest in Kulmbach, Bavaria, Germany (factorial ANOVA test at α = 0.05, n = 4). P-values ≤ 0.05 indicate a significant effect. Error bars show the standard error of the mean
Soil enzyme kinetics
Deadwood significantly increased the soil β-glucosidase activity by 136% and 44% at 0–4 cm and 8–12 cm depths, respectively (Fig. 3B). Deadwood significantly reduced the chitinase activity and enhanced the β-glucosidase efficiency only at 0–4 cm depth by 55% and 52%, respectively (Fig. 3C, Table 1). The activity and efficiency of cellobiosidase and the efficiency of chitinase were not significantly affected by deadwood at any depth (Fig. 3D, Table 1).
Soil microbial biomass
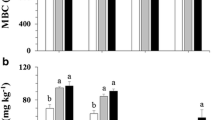
Deadwood significantly increased the soil MBC at 0–4 cm and 8–12 cm depths (Fig. 4A). Deadwood-induced 71% and 92% higher MBC at 0–4 cm and 8–12 cm depths, respectively. Deadwood also significantly enhanced soil MBN by 106% and 125% at 0–4 cm and 8–12 cm depths, respectively (Fig. 4B). The soil MBC to MBN ratio was not significantly affected by deadwood at any depth (Fig. 4C). The contribution of the MBC to SOC contents were significantly increased by deadwood up to 12% and 21% at 0–4 cm and 8–12 cm depths, respectively (Fig. 4D).
Effect of thinning-derived deadwood on the soil microbial biomass carbon (MBC) content (A), microbial biomass nitrogen (MBN) content (B), microbial biomass carbon to microbial biomass nitrogen ratio (MBC:MBN) (C), and microbial biomass carbon in soil organic carbon expressed in % (D) of the studied temperate spruce forest in Kulmbach, Bavaria, Germany (factorial ANOVA test at α = 0.05, n = 4). P-values ≤ 0.05 indicate a significant effect. Error bars show the standard error of the mean
Correlations between the measured variables
The SOC positively correlated with the total N (Pearson’s r = 0.97), C:N (r = 0.86), MBC (r = 0.93), MBN (r = 0.90), and β-glucosidase activity (r = 0.67), but negatively correlated with the extractable C/SOC (r =− 0.73) and MBC:MBN (r =− 0.70) (Table 2). Moreover, the soil MBC positively correlated with the extractable C (r = 0.97), MBN (r = 0.89), and β-glucosidase activity (r = 0.70), and the MBN negatively correlated with MB-C/N (r =—0.79). The MBC/SOC had a positive correlation with the extractable C/SOC (r = 0.62). There was a positive correlation between the β-glucosidase activity and extractable C (r = 0.68), and between the β-glucosidase and cellobiosidase activities (r = 0.75).
Discussion
Thinning-derived deadwood impact on the soil chemical properties
The overall low soil EC values suggest intense leaching of nutrients, contributing to the observed low soil pH. This is not surprising considering that the studied soil is a loamy sand Podzol located in a cool humid area with high precipitation amounts. Deadwood did not significantly affect the soil pH and EC. Whit-rot fungi, which were visually identified on the spruce deadwood in this study, degrade deadwood to lignin, cellulose, and small soluble byproducts enriched with carboxyl groups like calcium pectate (Schwarze 2007; Klotzbücher et al. 2013). Such carboxyl-enriched byproducts tend to adsorb hydroxyl-aluminum in the soil without liberating a lot of H+ (Thomas and Hargrove 1984), making the hydroxyl-aluminum hardly exchangeable and therefore decreasing the share of SOC in changing the soil EC and pH (Northup et al. 1998; Thomas and Hargrove 1984). The positive and negative correlations of SOC with EC (r = 0.74) and pH (r =− 0.62) indicate the contribution of the SOC to the EC and pH, although this contribution has not significantly changed the EC and pH values.
Deadwood increased the SOC contents by 59% and 56% at 0–4 cm and 8–12 cm depths, respectively. Even though the enhancements were not statistically significant, they indicate a strong increasing trend of the SOC under deadwood (p = 0.058) but contrast our main hypothesis. These increases in the SOC could be attributed to the soil type (Podzol on silicate bedrock) that has low pH, low nutrient contents, low biological activity, and slow SOC decomposition. Several mechanisms could have led to the enhanced SOC contents in the present study.
First, deadwood-derived POM could be transported into the soil through bioturbation. It is widely known that deadwood benefits soil meso-and macrofauna with improved micro-climatic conditions and nutrient availability; even in acidic soils with low biological activity (Jabin et al. 2004; Kappes et al. 2007; Huhta et al. 2012; Stutz et al. 2017; Wambsganss et al. 2017). Increased soil bioturbation under deadwood could promote accumulation of deadwood fragments, instead of their decomposition and respiration to the atmosphere. The overall low enzyme activities in the studied soil compared to those in the soils of other temperate forests (Bell and Henry 2011; DeForest 2009; Weand et al. 2010) support this finding. The negative correlation of the SOC content with the extractable C/SOC (r =− 0.73) further confirms this assumption, as it is an indicator of SOM decomposition (Ostrowska and Porębska 2015).
Second, deadwood-decaying fungi could have extended their mycelia into the soil and increased aggregate formation and stability by producing extracellular polymeric substances (EPS) (Caesar-TonThat 2002; Cairney 2005; Wambsganss et al. 2017). Specifically, white-rot fungi, which were identified on the deadwood in the present study, produce a lot of polysaccharide-rich EPS that play an important role in soil aggregation (Tisdall and Oades 1982; Eriksson et al. 1990; Caesar-Tonthat, 2002). Fungal hyphae could also translocate C compounds of the deadwood into the soil (Hughes and Boddy 1994; Frey et al. 2003; Boberg et al. 2010). This assumption is supported by the fact that cool and moist north-facing sites with low pH, like in the present study, are characterized by very high wood-decomposing fungal abundance and activity (Herrmann and Bauhus 2012; Fravolini et al. 2016; Gómez-Brandón et al. 2017a).
Third, deadwood may have stimulated litter decomposition and incorporation of its C compounds into the soil through increased microbial and faunal activity underneath. It was previously reported that the microbial decay of litter under spruce deadwood was done more rapidly than the litter without deadwood (Peršoh and Borken 2017). Each of these three mechanisms could have played a part in enhancing the SOC contents.
Deadwood did not significantly increase the soil total N contents at any depth. This seems logical considering that deadwood is a highly N-poor substrate (C:N = 350–800) (Vestin et al. 2013; Hoppe et al. 2014). The soil C:N was significantly higher under the deadwood at both depths compared to the controls. C:N is an indicator of SOM stability (Lützow et al. 2006; Ostrowska and Porębska 2015); the higher the C:N, the higher the stability of the SOM. Since Podzols are characterized by low biological activity, the addition of POM to the soil from the deadwood could have outweighed the decomposition by the soil microbial decomposers and therefore the POM has accumulated. Another reason for the increased C:N could have been the activity of fungi (Stutz and Lang 2017) and fauna in the soil beneath the deadwood, stabilizing soil aggregates and locking the SOC away from microbial decomposition. For example, fungal EPS could glue translocated POM by fauna into mineral particles of the soil, forming stable aggregates. These assumptions are consistent with the negative correlation between the C:N and SOC decomposition indicated by the extractable C/SOC (r =− 0.52). Ostrowska and Porębska (2015) also found a significant negative relationship between the C:N and extractable C/SOC, suggesting that the solubility of SOM is reduced by increasing the C:N. Moreover, translocated POM from the deadwood might have formed organo-mineral complexes in the presence of metal oxides, such as aluminum and iron (Baldock and Skjemstad 2000; Nazari et al. 2021), which are abundant in Podzols.
Deadwood significantly enhanced the soil extractable C, which is a proxy of DOC (Gonzalez-Polo et al. 2013; Wambsganss et al. 2017; Nazari et al. 2020). A reason for the increased DOC could be its direct translocation from the deadwood into the soil through precipitation water. The coarse soil texture (loamy sand) and high annual precipitation at the studied site as well as the role of decomposing deadwood in increasing soil porosity on silicate parent material (Sarker et al. 2018; Stutz et al. 2019) augments this conjecture. In agreement with these results, other studies also reported large amounts of DOC released from deadwood of different tree species (e.g., Hafner et al. 2005; Kahl et al. 2012; Gonzalez-Polo et al. 2013; Angst et al. 2017). DOC from deadwood can improve soil aggregate formation and stability, leading to enhanced SOC content (Spears and Lajtha 2004; Crow et al. 2007; Kappes et al. 2007; Gonzalez-Polo et al. 2013), which is confirmed by the strong correlation between the extractable C and SOC (r = 0.97) in the present study. This contradicts the results of Wambsganss et al (2017) that the leaching of DOC from deadwood does not affect the SOC content. The contrasting findings could have been related to the type of deadwoods and soils.
The share of the extractable C fraction in the SOC (an indicator of SOM decomposition) was significantly affected only by depth rather than by the presence of deadwood, being 30% greater at 8–12 cm depth than at 0–4 cm depth. A similar trend was observed in the study of Ostrowska and Porębska (2015). The negative correlations of the extractable C/SOC with the SOC (r =− 0.73) and C:N (r =− 0.52) and also the significant reductions in the SOC and C:N by depth imply an increased microbial decomposition of the SOC, due to decreased SOC stability by depth. A positive relationship (r = 0.62) between the soil extractable C/SOC and MBC/SOC (an indicator of substrate availability to microorganisms) further confirms this opinion. However, the increased microbial decomposition of the SOC probably costs the utilization of the soil N resources. This is indicated by a strong negative correlation between the soil extractable C/SOC and total N (r =− 0.74), which can worsen the already-existing N deficiency in the studied soil.
Thinning-derived deadwood impact on the soil enzyme kinetics
The enzyme activities in this study were generally low compared to those in the soils of other temperate forests (Bell and Henry 2011; DeForest 2009; Weand et al. 2010), resulting from the low fertility and in consequence low biological activity in Podzol soils. However, deadwood significantly increased the soil β-glucosidase activity at both depths, which is in line with our main hypothesis. Given the fact that spruce deadwood consists of approximately 40% cellulose (Petrillo et al. 2016) and that basidiomycetous fungi causing white-rot (visually observed on the deadwood) produce mainly cellulose-degrading enzymes like β-glucosidase (Baldrian 2006; Hatakka and Hammel 2010; Hayano and Tubaki 1985), the enhanced β-glucosidase activity in the soil for the degradation of organic matter coming from the deadwood is logical. This is consistent with the positive correlation between the β-glucosidase activity and the SOC content (r = 0.67). Wojciech et al. (2019) also reported that the increased activity of β-glucosidase in the soil under deadwood is associated with a high activity of wood-decomposing fungi. Furthermore, the translocation of DOC from the deadwood into the soil, as an energy source for microorganisms, might have induced the production of β-glucosidase (Peršoh and Borken 2017), which is verified by the positive correlation between the β-glucosidase activity and extractable C content (r = 0.68). The accumulation of SOM and products derived from deadwood decomposition in the soil provides microorganisms with easily degradable resources, leading to enhancements in the activity of extracellular enzymes like β-glucosidase (Wojciech et al. 2019). Furthermore, increased tree rooting and root exudates in the soil under deadwood (Kotroczo´ et al. 2014) could have also contributed to the higher β-glucosidase activity.
The β-glucosidase efficiency increased by about 50% at 0–4 cm depth. Although there was no significant correlation between the β-glucosidase efficiency and the other measured variables, we can link it to the higher C:N at 0–4 cm depth. This suggests that microorganisms in the soil might have invested in the production of β-glucosidase of higher efficiency to more easily breakdown the more stable SOM. The activity and efficiency of cellobiosidase were not significantly changed by deadwood at any depth. It could have been linked to the decay stage (age) of the deadwood at which microorganisms (i.e., fungi) in the soil preferentially produced more β-glucosidase than cellobiosidase for the degradation of the deadwood derivates. However, the positive correlation between the β-glucosidase and cellobiosidase activities (r = 0.75) implies their synergistic effect and co-metabolism. Deadwood significantly decreased the chitinase activity at 0–4 cm depth by nearly 50%. Chitinase is an N-degrading enzyme that works on fungal and insect detritus. The lower chitinase activity may have been associated with the preferential degradation of C-containing deadwood derivates rather than chitin-containing substrates in the soil.
Thinning-derived deadwood impact on the soil microbial biomass
In line with our main hypothesis, deadwood significantly increased the soil MBC. The enhanced MBC under deadwood could have resulted from several processes. First, DOC from the deadwood could have entered the soil through precipitation water and utilized by microorganisms (Rochette and Angers 2000; Allison and Martiny 2008). The strong positive correlation between the soil MBC and extractable C (r = 0.97) suggests that deadwood-derived DOC is a C source for microbial growth. Second, since deadwood, especially of spruce log, is poor in N and P, the growth of fungal hyphae from the deadwood into the soil for acquiring these minerals (Watkinson et al. 2006; Petrillo et al. 2016; Peršoh and Borken 2017; van der Wa et al. 2017) could have increased the soil fungal biomass and thereby the MBC. Finally, organic matter from deadwood is a substrate for microbial utilization (Chen and Xu 2005; Wang and Wang 2007) and therefore has enhanced the MBC. However, the positive correlation between the MBC and SOC (r = 0.93) and the lack of a significant correlation between the MBC/SOC (an indicator of substrate availability) and SOC demonstrate an indirect effect of SOC on the MBC (e.g., through fungal hyphae and EPS). The MBC/SOC contents were increased by deadwood, indicating that deadwood has enhanced the availability of substrates to the soil microorganisms. However, the non-significant correlation of the MBC with the MBC/SOC indicates that the C derived from the SOC though decomposition, despite availability, has not been accessible to microorganisms.
Deadwood significantly increased the soil MBN by 105.5% and 124.7% at 0–4 cm and 8–12 cm depths, respectively. The negative correlation between the MBN and extractable C/SOC (r =− 0.51) and the lack of significant correlation between the MBN and MBC/SOC indicate that the MBN has not derived from the microbial decomposition of the SOC. It is possible that, in addition to the stabilization of the SOC, EPS derived from abundant fungal mycelia in the soil beneath the deadwood have provided optimum conditions (i.e., < 5% oxygen) for bacterial N fixation (Wessel et al. 2014; Wang et al. 2017a, b). Moreover, it was indicated that bacterial N fixation occurs in living sporocarps of deadwood-decomposing fungi to compensate for their N deficiency (Larsen et al. 1978; Hoppe et al. 2014). The inherent N deficiency of the studied soil and the fact that deadwood is a highly N-poor substrate (Vestin et al. 2013; Hoppe et al. 2014) make the bacterial fixation of N from the atmosphere possible. The energy for bacterial N fixation could have been supplied by the DOC derived from the deadwood, indicated by the strong positive correlation between the MBN and extractable C (r = 0.94).
The soil MBC:MBN was not significantly influenced by deadwood at any depth but was significantly higher at 0–4 cm than at 8–12 cm depth. MBC:MBN is an indicator of N limitation for soil microorganisms (Joergensen and Emmerling 2006; Hartman and Richardson 2013; Nazari et al. 2020). The high MBC:MBN values in the present study imply that microorganisms in the soil have been strongly limited with N. The negative correlation of the MBN with the MBC:MBN (r =−0.79) supports this viewpoint. The N limitation in the soil could have been caused by the translocation of N from the soil into the deadwood through foraging hyphae of fungi inhabiting the deadwood (Watkinson et al. 2006; van der Wa et al. 2017). This is consistent with the generally low N content of deadwood (Vestin et al. 2013; Petrillo et al. 2016) and the importance of N for fungi to build up their chitin cell wall and proteins (Purahong et al. 2016) as well as the negative correlation between the soil MBC:MBN and total N.
Conclusion
For the first time, this study evaluated the effect of thinning-derived spruce deadwood logs on the soil chemical and microbial properties of an acidic loamy sand Podzol in a temperate spruce forest. Although not significantly, the strong trend of higher SOC contents suggests that keeping slow-decaying deadwood like spruce logs on soils of slow biogeochemical cycles like Podzol can be an appropriate management strategy for C sequestration. Slow decomposition of deadwood over years promotes the accumulation of considerable C stocks in soil rather than C mineralization to the atmosphere as CO2. Deadwood can also function as an important source of C for soil biota, which can in the long-term elevate the soil fertility (i.e., increased MBC and enzyme activity). Since Podzols generally underlay forests, maintenance of thinning-derived deadwood logs on the soil can support soil and forest sustainability as well as C sequestration.
Data Availability
For information about the data and material, please contact the corresponding author.
Code availability
Not applicable.
References
Allison SD, Martiny JB (2008) Resistance, resilience, and redundancy in microbial communities. Proc Natl Acad Sci 105:11512–11519
Anderson TH, Domsch KH (1993) The metabolic quotient for CO2 (qCO2) as a specific activity parameter to assess the effects of environmental conditions, such as pH, on the microbial biomass of forest soils. Soil Biol Biochem 25:393–395
Angst G, Mueller KE, Kögel-Knabner I, Freeman KH, Mueller CW (2017) Aggregation controls the stability of lignin and lipids in clay-sized particulate and mineral associated organic matter. Biogeochemistry 132(3):307–324. https://doi.org/10.1007/s10533-017-0304-2
Bade C, Jacob M, Leuschner C, Hauck M (2015) Chemical properties of decaying wood in an old-growth spruce forest and effects on soil chemistry. Biogeochemistry 122:1–13. https://doi.org/10.1007/s10533-014-0015-x
Baldock JA, Skjemstad JO (2000) Role of the soil matrix and minerals in protecting natural organic materials against biological attack. Org Geochem 31:697–710
Baldrian P (2006) Fungal laccases-occurrence and properties. FEMS Microbiol Rev 30:215–242
Bastin J-F, Berrahmouni N, Grainger A, Maniatis D, Mollicone D, Moore R, Patriarca C, Picard N, Sparrow B, Abraham EM (2017) The extent of forest in dryland biomes. Science 356:635–638
Bell TH, Henry HAL (2011) Fine scale variability in soil extracellular enzyme activity is insensitive to rain events and temperature in a mesic system. Pedobiologia 54:141–146. https://doi.org/10.1016/j.pedobi.2010.12.003
Boberg JB, Finlay RD, Stenlid J, Lindahl BD (2010) Fungal C translocation restricts N mineralization in heterogeneous environments. Funct Ecology 24:e454–e459
Bonan GB (2008) Forests and climate change: forcings, feedbacks, and the climate benefits of forests. Science 320:1444–1449. https://doi.org/10.1126/science.1155121
Brookes PC, Landman A, Pruden G, Jenkinson DS (1985) Chloroform fumigation and the release of soil nitrogen: a rapid direct extraction method for measuring microbial biomass nitrogen in soil. Soil Biol Biochem 17:837–842
Caesar-Tonthat TC (2002) Soil binding properties of mucilage produced by a basidiomycete fungus in a model system. Mycol Res 106:930–937. https://doi.org/10.1017/S0953756202006330
Cairney JWG (2005) Basidiomycete mycelia in forest soils: dimensions, dynamics and roles in nutrient distribution. Mycol Res 109:7–20. https://doi.org/10.1017/S0953756204001753
Chang C, Fuzhong W, Yang W, Zhenfeng X, Cao R, He W, Tan B, Justine MF (2017) The microbial community in decaying fallen logs varies with critical period in an alpine forest. PLoS ONE 12(8):e0182576. https://doi.org/10.1371/journal.pone.0182576
Chen CR, Xu ZH, Mathers NJ (2004) Soil carbon pools in adjacent natural and plantation forests of subtropical Australia. Soil Sci Soc Am J 68:282–291
Crow SE, Swanston CW, Lajtha K, Brooks JR, Keirstead H (2007) Density fractionation of forest soils: methodological questions and interpretation of incubation results and turnover time in an ecosystem context. Biogeochemistry 85:69–90
DeForest JL (2009) The influence of time, storage temperature, and substrate age on potential soil enzyme activity in acidic forest soils using MUB-linked substrates and l-DOPA. Soil Biol Biochem 41:1180–1186. https://doi.org/10.1016/j.soilbio.2009.02.029
Eriksson E, Gillespie AR, Gustavsson L, Langvall O, Olsson M, Sathre R, Stendahl J (2007) Integrated carbon analysis of forest management practices and wood substitution. Can J Forest Res 37:671–681. https://doi.org/10.1139/X06-257
Fravolini G, Egli M, Derungs C, Cherubini P, Ascher-Jenull J, Gómez Brandón M, Bardelli T, Tognetti R, Lombardi F, Marchetti M (2016) Soil attributes and microclimate are important drivers of initial deadwood decay in sub-alpine Norway spruce forests. Sci Tot Environ 569–570:1064–1076
Frey SD, Six J, Elliott ET (2003) Reciprocal transfer of carbon and nitrogen by decomposer fungi at the soilelitter interface. Soil Biol Biochem 35:e1001–e1004
Goldin SR, Hutchinson MF (2013) Coarse woody debris modifies surface soils of degraded temperate eucalypt woodlands. Plant Soil 370:461–469. https://doi.org/10.1007/s11104-013-1642-z
Gómez-Brandón M, Ascher-Jenull J, Bardelli T, Fornasier F, Fravolini G, Arfaioli P, Ceccherini MT et al (2017) Physico-chemical and microbiological evidence of exposure effects on picea abies – coarse woody debris at different stages of decay. Forest Ecol Manag. https://doi.org/10.1016/j.foreco.2017.02.033
Gómez-Brandón M, Ascher-Jenull J, Bardelli T, Fornasier F, Sartori G, Pietramellara G, Arfaioli P et al (2017) Ground cover and slope exposure effects on micro- and mesobiota in forest soils. Ecolog Indicat. https://doi.org/10.1016/j.ecolind.2017.05.032
Gonzalez-Polo M, Fernández-Souto A, Austin AT (2013) Coarse woody debris stimulates soil enzymatic activity and litter decomposition in an old-growth temperate forest of Patagonia, Argentina. Ecosystems 16:1025–1038. https://doi.org/10.1007/s10021-013-9665-0
Grove S (2001) Extent and composition of dead wood in Australian lowland tropical rainforest with different management histories. For Ecol Manage 154:35–53
Hafner SD, Groffman PM, Mitchell MJ (2005) Leaching of dissolved organic carbon, dissolved organic nitrogen, and other solutes from coarse woody debris and litter in a mixed forest in New York State. Biogeochemistry 74:257–282. https://doi.org/10.1007/s10533-004-4722-6
Hartman WH, Richardson CJ (2013) Differential nutrient limitation of soil microbial biomass and metabolic quotients (qCO2): is there a biological stoichiometry of soil microbes? PLoS ONE 8:e57127
Hatakka A, Hammel KE (2010) Fungal biodegradation of lignocelluloses. In: Osiewacz HD (ed) The Mycota: a comprehensive treatise on fungi as experimental systems for basic and applied research. Springer, Berlin, pp 319–340
Hayano K, Tubaki K (1985) Origin and properties of β-glucosidase activity of tomatofield soil. Soil Biol Biochem 17:553–557
Herrmann S, Bauhus J (2012) Effects of moisture, temperature and decomposition stage on respirational carbon loss from coarse woody debris (CWD) of important European tree species. Scand J Forest Res 28:346–357
Hoppe B, Kahl T, Karasch P, Wubet T, Bauhus J, Buscot F, Krüger D (2014) Network analysis reveals ecological links between N-fixing bacteria and wooddecaying fungi. PLoS ONE 10:e88141
Hughes CL, Boddy L (1994) Translocation of P-32 between wood resources recently colonized by mycelial cord systems of Phanerochaete velutina. FEMS Microbiol Ecol 14:e201–e212
Huhta V, Siira-Pietikäinen A, Penttinen R (2012) Importance of dead wood for soil mite (Acarina) communities in boreal old-growth forests. Soil Organisms 84(3):499–512
IPCC (2007) Climate Change 2007: The physical science basis contribution of working group I to the fourth assessment report of the intergovernmental panel on climate. Change. Cambridge University Press, Cambridge, UK and New York, NY, USA. pp 10
Jabin M, Mohr D, Kappes H, Topp W (2004) Influence of deadwood on density of soil macro-arthropods in a managed oak-beech forest. Forest Ecol Manage 194:61–69. https://doi.org/10.1016/j.foreco.2004.01.053
James J, Harrison R (2016) The effect of harvest on forest soil carbon: A meta-analysis. Forests 7:308
Jandl R, Lindner M, Vesterdal L, Bauwens B, Baritz R, Hagedorn F, Johnson DW, Minkkinen K, Byrne KA (2007) How strongly can forest management influence soil carbon sequestration? Geoderma 137:253–268
Joergensen RG, Emmerling C (2006) Methods for evaluating human impact on soil microorganisms based on their activity, biomass, and diversity in agricultural soils. J Plant Nutr Soil Sci 169:295–309
Kahl T, Mund M, Bauhus J, Schulze E (2012) Dissolved organic carbon from European beech logs: patterns of input to and retention by surface soil. Ecoscience 19:1–10
Kappes H, Catalano C, Topp W (2007) Coarse woody debris ameliorates chemical and biotic soil parameters of acidified broad-leaved forests. Appl Soil Ecol 36:190–198. https://doi.org/10.1016/j.apsoil.2007.02.003
Keenan RJ, Reams GA, Achard F, de Freitas JV, Grainger A, Lindquist E (2015) Dynamics of global forest area: results from the FAO global forest resources assessment 2015. For Ecol Manage 352:9–20
Klotzbücher T, Kaiser K, Filley TR, Kalbitz K (2013) Processes controlling the production of aromatic water-soluble organic matter during litter decomposition. Soil Biol Biochem 67:133–139. https://doi.org/10.1016/j.soilbio.2013.08.003
Kotroczo´, Z., Veres, Z., Fekete, J´, et al (2014) Soil enzyme activity in response to long-term organic matter manipulation. Soil Biol Biochem 70:237–243
Krzyszowska-Waitkus A, Vance GF, Preston CM (2006) Influence of coarse wood and fine litter on forest organic matter composition. Can J Soil Sci 86:35–46. https://doi.org/10.4141/S05-040
Lachat T, Brang P, Bolliger M, Bollmann K, Brändli U-B, Bätler R, Hermann S, Schneider O, Wermelinger B (2014) Totholz im Wald Entstehung, Bedeutung und Färderung. Merkbl Prax 52:12S
Laganiere J, Angers DA, Pare D (2010) Carbon accumulation in agricultural soils after afforestation: a meta-analysis. Glob Change Biol 16:439–453
Larsen MJ, Jurgensen MF, Harvey AE (1978) Nitrogen fixation associated with wood decayed by some common fungi in western Montana. Can J for Res 8:341–345
Lombardi F, Cherubini P, Tognetti R, Cocozza C, Lasserre B, Marchetti M (2013) Investigating biochemical processes to assess deadwood decay of beech and silver fir in mediterranean mountain forests. Ann for Sci 70:101–111. https://doi.org/10.1007/s13595012-0230-3
Lützow M, Kogel-Knabner I, Ekschmitt K, Matzner E, Guggenberger G (2006) Stabilization of organic matter in temperate soils: mechanism and their relevance under different soil conditions–a review. Eur J Soil Sci 57:426
Ma Y, Filley TR, Szlavecz K, McCormick MK (2014) Controls on wood and leaf litter incorporation into soil fractions in forests at different successional stages. Soil Biol Biochem 69:212–222. https://doi.org/10.1016/j.soilbio.2013.10.043
Magnússon RI, Tietema A, Cornelissen JHC, Hefting MM, Kalbitz K (2016) Tamm review: sequestration of carbon from coarse woody debris in forest soils. Ecol Manag 377:1–15. https://doi.org/10.1016/j.foreco.2016.06.033
Mahmoud AY, Blagodatskaya E, Ok YS, Kuzyakov Y (2012) Effects of polyacrylamide, biopolymer, and biochar on decomposition of soil organic matter and plant residues as determined by 14C and enzyme activities. Europ J Soil Biology 48:1–10. https://doi.org/10.1016/j.ejsobi.2011.09.005
Makinen H, Isomaki A (2004) Thinning intensity and growth of Scots pine stands in Finland. For Ecol Manage 201:311–325
Marx MC, Kandeler E, Wood M, Wermbter N, Jarvis SC (2005) Exploring the enzymatic landscape: distribution and kinetics of hydrolytic enzymes in soil particle size fractions. Soil Biol Biochem 37:35–48. https://doi.org/10.1016/j.soilbio.2004.05.024
Mathias M, Cindy E, Prescott WE, Abaker LA, Cécillon L, Ferreira GWD, James J et al (2020) Influence of forest management activities on soil organic carbon stocks: a knowledge synthesis. Forest Ecol Manag. https://doi.org/10.1016/j.foreco.2020.118127
Meisam N, Horvat M, Joergensen RG, Peth S (2020) Soil organic matter mobilization by re-compaction of old forest skid trails. Europ J Soil Biol. https://doi.org/10.1016/j.ejsobi.2020.103173
Moreno-Fernández D, Díaz-Pinés E, Barbeito I, Sánchez-González M, Montes F, Rubio A, Cañellas I (2015) Temporal carbon dynamics over the rotation period of two alternative management systems in Mediterranean mountain scots pine forests. For Ecol Manage 348:186–195
MORRIS, D.M. (1997) The role of long-term site productivity in maintaining healthy ecosystems: a prerequisite of ecosystem management. For Chron 73:731–740
Mushinski RM, Gentry TJ, Boutton TW (2019) Forest organic matter removal leads to long-term reductions in bacterial and fungal abundance. Appl Soil Ecol 137:106–110
Nave LE, Vance ED, Swanston CW, Curtis PS (2010) Harvest impacts on soil carbon storage in temperate forests. For Ecol Manage 259:857–866
Nazari M, Eteghadipour M, Zarebanadkouki M, Ghorbani M, Dippold MA, Bilyera N, Zamanian K (2021) Impacts of logging-associated compaction on forest soils: a meta-analysis. Front for Glob Change 4:780074. https://doi.org/10.3389/ffgc.2021.780074
Neruda J, Kadlec J, Ulrich R, Cudzik A (2010) Soil carbon dioxide concentration and efflux changes in ruts after heavy machine passes. In: FORMEC 2010 Forest Engineering: Meeting the Needs of the Society and the Environment, Padova
Northup RR, Dahlgren RA, McColl JG (1998) Polyphenols as regulators of plantlitter-soil interactions in northern California’s pygmy forest: a positive feedback? Biogeochemistry 42:189–220. https://doi.org/10.1023/A:1005991908504
Ostrowska A, Porębska G (2015) Assessment of the C/N Ratio as an Indicator of the decomposability of organic matter in forest soils. Ecol Ind. https://doi.org/10.1016/j.ecolind.2014.09.044
Page-Dumroese DS, Jurgensen MF (2006) Soil carbon and nitrogen pools in mid- to late-successional forest stands of the northwestern USA: Potential impact of fire. Can. J Res 36:2270–2284
Page-Dumroese DS, Jurgensen M, Terry T (2010) Maintaining soil productivity during forest or biomass-to-energy thinning harvests in the western United States. West J Appl Forest. https://doi.org/10.1093/wjaf/25.1.5
Pan Y, Birdsey RA, Fang J, Houghton R, Kauppi PE, Kurz WA, Phillips OL, Shvidenko A, Lewis SL, Canadell JG, Ciais P, Jackson RB, Pacala SW, McGuire AD, Piao S, Rautiainen A, Sitch S, Hayes D (2011) A large and persistent carbon sink in the world’s forests. Science 333:988–993
Perreault L, Forrester JA, Wurzburger N, Mladenoff DJ (2020) Emergent properties of downed woody debris in canopy gaps: a response of the soil ecosystem to manipulation of forest structure. Soil Biol Biochem. https://doi.org/10.1016/j.soilbio.2020.108053
Peršoh D, Borken W (2017) Impact of woody debris of different tree species on the microbial activity and community of an underlying organic horizon. Soil Biol Biochem. https://doi.org/10.1016/j.soilbio.2017.09.017
Petrillo M, Cherubini P, Fravolini G, Marchetti M, Ascher-Jenull J, Schärer M, Synal HA, Bertoldi D, Camin F, Larcher R, Egli M (2016) Time since death and decay rate constants of Norway spruce and European larch deadwood in subalpine forests determined using dendrochronology and radiocarbon dating. Biogeosciences 13:1537–1552
Powers M, Kolka R, Palik B, McDonald R, Jurgensen M (2011) Long-term management impacts on carbon storage in lake states forests. For Ecol Manage 262:424–431
Purahong W, Arnstadt T, Kahl T, Bauhus J, Kellner H, Hofrichter M, Krüger D, Buscot F, Hoppe B (2016) Are correlations between deadwood fungal community structure, wood physico-chemical properties and ligninmodifying enzymes stable across different geographical regions? Fungal Ecol 22:98–105
Rochette P, Angers DA (2000) Soil carbon and nitrogen dynamics following application of pig slurry for the 19th consecutive year i. Carbon dioxide fluxes and microbial biomass carbon. Soil Sci Soc Am J 64:1389–1395
Sarker TC, Incerti G, Spaccini R, Piccolo A, Mazzoleni S, Bonanomi G (2018) Linking organic matter chemistry with soil aggregate stability: insight from 13C NMR spectroscopy. Soil Biol Biochem 117:175–184. https://doi.org/10.1016/j.soilbio.2017.11.011
Schwarze FWMR (2007) Wood decay under the microscope. Fungal Biol Rev 21:133–170. https://doi.org/10.1016/j.fbr.2007.09.001
Sohn JA, Saha S, Bauhus J (2016) Potential of forest thinning to mitigate drought stress: a meta-analysis. For Ecol Manage 380:261–273
Spears JDH, Lajtha K (2004) The imprint of coarse woody debris on soil chemistry in the western Oregon cascades. Biogeochemistry 71:163–175. https://doi.org/10.1007/s10533-0046395-6
Spears JDH, Holub SM, Harmon ME, Lajtha K (2003) The influence of decomposing logs on soil biology and nutrient cycling in an old-growth mixed coniferous forest in Oregon, U.S.A. Can J for Res 33:2193–2201. https://doi.org/10.1139/x03-148
Ministerial Conference on the Protection of Forests in Europe (2015) State of Europe’s Forests. Technical Report. http://www.foresteurope.org/docs/fullsoef2015.pdf.
Stutz KP, Lang F (2017) Potentials and unknowns in managing coarse woody debris for soil functioning. Forests 8:37
Stutz KP, Dann D, Wambsganss J, Scherer-Lorenzen M, Lang F (2017) Phenolic matter from deadwood can impact forest soil properties. Geoderma. https://doi.org/10.1016/j.geoderma.2016.11.014
Stutz KP, Kaiser K, Wambsganss J, Santos F, Berhe AA, Lang F (2019) Lignin from white-rotted european beech deadwood and soil functions. Biogeochemistry. https://doi.org/10.1007/s10533-019-00593-2
Thomas GW, Hargrove WL (1984) The chemistry of soil acidity. In: Adams F (ed) Soil acidity and liming agron monogr. American Society of Agronomy, Crop Science Society of America Soil Science Society of America, Madison WI, pp 3–56
Tisdall J, Oades J (1982) Organic matter and water-stable aggregates in soils. J Soil Sci 33:141–163. https://doi.org/10.1111/j.1365-2389.1982.tb01755.x
van der Wal A, Paulien KG, de Wietse B (2017) Soil-wood interactions: influence of decaying coniferous and broadleaf logs on composition of soil fungal communities. Fungal Ecol 30:132–134. https://doi.org/10.1016/j.funeco.2017.08.006
Vesterdal L, Dalsgaard M, Felby C, Raulund-Rasmussen K, Jørgensen BB (1995) Effects of thinning and soil properties on accumulation of carbon, nitrogen and phosphorus in the forest floor of Norway spruce stands. For Ecol Manage 77:1–10
Vestin JLK, Söderberg U, Bylund D, Nambu K, Hees PAW, Haslinger E, Ottner F, Lundström US (2013) The influence of alkaline and non-alkaline parent material on Norway spruce tree chemical composition and growth rate. Plant Soil 370:103–113
Wambsganss J, Stutz KP, Lang F (2017) European beech deadwood can increase soil organic carbon sequestration in forest Topsoils. For Ecol Manage. https://doi.org/10.1016/j.foreco.2017.08.053
Wang QK, Wang SL (2007) Soil organic matter under different forest types in Southern China. Geoderma 142:34–56
Wang D, Xu A, Elmerich C, Ma LZ (2017a) Biofilm formation enables free-living nitrogen-fixing rhizobacteria to fix nitrogen under aerobic conditions. ISME J 11:1602–1613. https://doi.org/10.1038/ismej.2017.30
Wang J, Yan Q, Yan T, Song Y, Sun Y, Zhu J (2017b) Rodent-mediated seed dispersal of Juglans mandshurica regulated by gap size and within-gap position in larch plantations: implication for converting pure larch plantations into larch-walnut mixed forests. For Ecol Manage 404:205–213
Watkinson S, Bebber D, Darrah P, Fricker M, Tlalka M, Boddy L (2006) The role of wood decay fungi in the carbon and nitrogen dynamics of the forest floor. In: Gadd GM (ed) Fungi in Biogeochemical Cycles. Cambridge University Press, Cambridge, pp e151–e181
Weand MP, Arthur MA, Lovett GM, McCulley RL, Weathers KC (2010) Effects of tree species and N additions on forest floor microbial communities and extracellular enzyme activities. Soil Biol Biochem 42:2161–2173. https://doi.org/10.1016/j.soilbio.2010.08.012
Wei X, Shao M, Gale W, Li L (2014) Global pattern of soil carbon losses due to the conversion of forests to agricultural land. Sci Rep 4:4062
Wessel AK, Arshad TA, Fitzpatrick M, Connell JL, Bonnecaze RT, Shear JB et al (2014) Oxygen limitation within a bacterial aggregate. Mbio 5:e00992. https://doi.org/10.1128/mBio.00992-14
Wojciech P, Ewa B, Jarosław L (2013) Soil biochemical properties and stabilisation of soil organic matter in relation to deadwood of different species. FEMS 95(3):11. https://doi.org/10.1093/femsec/fiz011
Wu J, Joergensen RG, Pommerening B, Chaussod R, Brookes PC (1990) Measurement of soil microbial biomass-C by fumigation-extraction – an automated procedure, Soil Biol. Biochem 22:1167–1169
Zalamea M, González G, Ping C-L, Michaelson G (2007) Soil organic matter dynamics under decaying wood in a subtropical wet forest: effect of tree species and decay stage. Plant Soil 296:173–185. https://doi.org/10.1007/s11104-007-9307-4
Zhang B, Zhou X, Zhou L, Ju R (2015) A global synthesis of below-ground carbon responses to biotic disturbance: a meta-analysis. Glob Ecol Biogeogr 24:126–138
Zhang X, Guan D, Li W, Sun D, Jin C, Yuan F, Wang A, Wu J (2018) The effects of forest thinning on soil carbon stocks and dynamics: A meta-analysis. For Ecol Manage 429:36–43
Zhou D, Zhao S, Liu S, Oeding J (2013) A meta-analysis on the impacts of partial cutting on forest structure and carbon storage. Biogeosciences 10:3691–3703
Acknowledgements
We would like to thank Mr. Kurt Sollmann for kindly thinning the stand, Ms. Ilse Thaufelder for her kind laboratory assistance, and Mr. Andreas Kolb for determining the soil texture. We also acknowledge the German Federal Environmental Foundation for funding the Ph.D. studies of Meisam Nazari during the preparation of the manuscript.
Funding
Open Access funding enabled and organized by Projekt DEAL. Not applicable.
Author information
Authors and Affiliations
Contributions
M.N proposed the idea, designed the experiment, measured the soil biochemical characteristics, did the statistical analyses, and wrote the manuscript draft; M.R. and B.R. measured the enzyme kinetics; M.Z. and J. P. supervised the study. S.B, N.B, K.Z., A.S., L.S, and M.D scientifically contributed to the discussion and writing of the manuscript draft.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Conflicts of interest
The authors declare no conflicts of interest.
Additional information
Communicated by Agustin Merino.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Nazari, M., Pausch, J., Bickel, S. et al. Keeping thinning-derived deadwood logs on forest floor improves soil organic carbon, microbial biomass, and enzyme activity in a temperate spruce forest. Eur J Forest Res 142, 287–300 (2023). https://doi.org/10.1007/s10342-022-01522-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10342-022-01522-z