Abstract
During mechanized forest operations, machines with high wheel loads are operated directly on the soil and can pose serious concerns for the integrity and quality of soil properties. Simply relying on absolute soil density (g cm−3 or t m−3) as a measure of qualitative assessment remains site specific. Instead of absolute soil density, the aim of this study was to assess and quantify the above- and belowground biomass growth response of Norway spruce (Picea abies [L.] Karst) seedlings exposed to varying relative bulk densities (RBD), expressed as a ratio between field bulk density and maximum bulk density achieved through the standard Proctor test. In the laboratory study, silt loam collected from a forest stand located near Munich, Germany, was placed inside 24 chambers (30.6 cm in length, 8.2 cm in width, and 42.0 cm in height) and compacted layerwise with a hydraulic press until the target RBD levels (0.67, 0.72, 0.77, and 0.82) were reached. After compaction, 120 Norway spruce seeds were sown and growth was monitored for 156 days. Morphologic measurements of 102 seedlings were performed after the growing period where root, shoot, and needle growth characteristics and mass were recorded. Detailed root analysis was performed with the WinRHIZO software, and nutrient analysis was performed on the soil as well as the root, shoot, and needle components of seedlings. Results indicate statistically lower average root (48%), shoot (43%), and needle (43%) mass per seedling for seedlings growing on the RBD 0.82 as compared to those growing on the RBD 0.67. Most analyses revealed no statistical differences between the two lower RBD levels, while statistical differences were detected as the RBD increased to 0.77 and 0.82. The use of RBD as a means of qualitative assessment of soil properties showed promise and should be investigated further for other economically important European tree species, while taking into consideration a longer monitoring period.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Forest soils are an integral and vital part of the forest ecosystem as ecological processes, which govern tree survival and growth, are concentrated around the soil and root interface (Coder 2016). In addition to providing anchorage substrate, forest soils also form a reservoir for available water and nutrients, while offering habitat for a diverse fauna (Schaefer and Schauermann 1990; Brady and Weil 1996; Binkley and Fisher 2013). In today’s forestry, management activities using heavy ground-based machines can lead to detrimental damages to soils such as compaction (Froehlich et al. 1986; Kozlowski 1999) and soil displacement (Poltorak et al. 2018). Many environmental constraints affect tree survival and growth but soil compaction, particularly in moist and wet soils, remains one of the most significant problems (Coder 2016). Compacted soils exhibit increased shear strength (Blackwell et al. 1986) and penetration resistance (Labelle et al. 2019), reduced pore volume and lowered air permeability (Costello et al. 1991; Ezzati et al. 2012; Fründ and Averdiek 2016), and thus ultimately affect tree growth (Greacen and Sands 1980; Naghdi et al. 2016). With the increased use of machines with high wheel loads, the frequency and magnitude of soil disturbances in forest operations are on the rise (Rohand et al. 2004). This problem threatens to be compounded by the effects of a changing climate where the majority of harvesting operations in Germany will be carried out in unfrozen soil conditions, thereby removing yet another natural mitigating technique of reducing machine-induced soil compaction.
During mechanized forest operations, machine operating trails are used to allow the movement and operation (felling, processing, and timber extraction) of forest machines throughout the harvest area. In Germany, the majority of the trail network was established 30–40 years ago when the frequency of mechanized harvesting was quite low. With advancements and refinements in machine design and harvesting systems, a redesign of the trail network has been justified and in many instances simply unavoidable. This implies that unlike common belief, the layout of machine operating trails in Germany is much less “permanent” than anticipated. As machine technical advancements continue to be refined and new guidelines are developed to better protect the physical environment, trail networks will likely continue to undergo modifications thereby exposing new, previously undisturbed areas, to direct machine traffic (Labelle and Jaeger 2019). Even in cases where average trail spacing is increasing (State of Baden Württemberg: from 20 to 40 m in public forests), new trails have to be created while others now need to support tree growth. Moreover, the rehabilitation of compacted soils by forest machines is a lengthy process that can take many years to several decades (Power 1974; Froehlich et al. 1986; Labelle and Jaeger 2011; Jourgholami et al. 2019), which implies that soils within abandoned machine operating trails can be damaged severely enough to continue causing tree growth impediments long after trail alterations are completed.
In addition to the spatial variability of machine operating trails, studies have shown that the impact of forest machines on soils is not limited to the area in direct contact with the machine running gear (tires or tracks) but extends laterally beyond the contact area (Labelle and Jaeger 2011; Labelle et al. 2019). Labelle and Jaeger (2011) measured soil compaction in the range of 10–15% up to 1.5 m away from tire contact area when compared to undisturbed areas. Considering an average tire width of 0.75 m and trail spacing of 30 m (center to center), the area impacted by a common forest machine increases from 500 to 2000 m2 ha−1, thus effectively quadrupling the disturbed area. Thus, operating machines directly on unprotected machine operating trails exposes at least 5% of the total treated forest area to direct machine traffic (tire contact area) and 20% when considering the indirect impact caused by lateral load distribution beyond tire footprint. This example remains very conservative because actual forest operations display varying trail spacing designed below the optimum alignment, and the tire width of today’s forest machines can exceed by far the 0.75 m as used in the above example. Because of these three important facts (operations performed on moist and unfrozen soils due to changing climate, trail repositioning, and area of disturbance), the effect of machine-induced soil compaction on seedling growth needs to be further investigated.
In the literature, soil compaction is usually expressed as a change in absolute soil density between pre- and post-harvest levels (Craig 2005; Han et al. 2009; Giannetti et al. 2017; Cambi et al. 2018). While the absolute density change is an important quantitative measure when monitoring soil disturbances, its significance with respect to available pore space following machine impact and associated biological activity is quite limited (da Silva et al. 1994). As an example, different soil types exposed to the same compactive energy (wheel load) can exhibit diverse effects on plant growth depending on the quality and quantity of the remaining pore space. This means that a 5% increase in soil bulk density on one site can be more problematic than a 20% increase on a different site, which makes results from published literature highly dependent on local soil conditions. To remedy this deficiency in the evaluation of soil compaction, the concept of “relative bulk density” (RBD) was developed and first applied in the field of geotechnical engineering. RBD (Eq. 1), which relates field bulk density (FBD) to maximum bulk density (MBD) obtained by a standardized test, the standard Proctor compaction test, is a more objective approach to quantify machine-induced soil compaction and the degree of compactness (Pidgeon and Soane 1977; Carter 1990; da Silva et al. 1994; Håkansson and Lipiec 2000; Lipiec and Håkansson 2000; Labelle and Jaeger 2011; Kormanek et al. 2015; Labelle et al. 2019). The strongest advantage of this method is the use of the worldwide-accepted standard Proctor test, an ASTM and DIN-approved quality assessment method for roadbed construction (ASTM D698 12e2; DIN 18127:2012-09). As reference, a RBD of 1.00 implies that FBD is equal to MBD as achieved by the standard Proctor test.
First tests of RBD beyond geotechnical engineering were performed by Carter (1990) on agricultural crops located in eastern Canada where a RBD greater than 0.80 was determined to be problematic for growth of corn. Following this agricultural study, Zhao et al. (2010) analyzed the effects of varying relative bulk densities of loam, sandy loam, and silty clay loam soils on Douglas fir (Pseudotsuga menziesii [Mirb.] Franco) seedling growth and confirmed that a RBD > 0.80 was also problematic for tree growth and should be considered as a marker for significant soil degradation. Under these challenging growing conditions, the soil microbial activity is significantly affected and the oxygen exchange rate is no longer sufficient to maintain unobstructed plant growth. More recently, Kormanek et al. (2015) reported statistically significant root growth and root system dry mass reductions of sessile oak (Quercus petraea [Matt.] Liebl.) seedlings when growing in soils beyond a RBD of 0.75. Tree growth obstructions resulting from machine disturbances are not trivial as Murphy et al. (2004) reported that reduced growth in a radiata pine (Pinus radiata D. Don) plantation, following subsoil compaction below a depth of 10 cm, resulted in a decrease of stand volume of up to a 42%, which translated to a 60% decline in value over a 28-year projection period. A loss in wood volume due to soil compaction does not only reduce the expected financial income of a harvesting operation but also implies lower carbon storage by the affected trees.
Despite some initial findings on the use of RBD as a means of identifying growth-impeding thresholds in a forestry context, no information is available for Norway spruce (Picea abies [L.] Karst), yet. The importance of Norway spruce for the German forest and timber industry is significant as it represents 43% of the forest area, 51% of the standing volume, and 66% of wood volume sold within forests owned by the Bavarian State Forest (Bayerische Staatsforsten AöR 2018). Because of the prominence of Norway spruce, this study attempted to gain new knowledge by assessing and quantifying the above- and belowground biomass response of Norway spruce seedlings exposed to varying levels of soil relative bulk densities (0.67, 0.72, 0.77, and 0.82). Detailed biomass measurements of the root, shoot, and needles of 102 seedlings were performed after a growing period of 156 days. The degree of mycorrhization was also analyzed for all seedlings.
Materials and methods
Experimental design
In this study, the above- and belowground growth of Norway spruce seedlings was monitored at four treatments of 0.67, 0.72, 0.77, and 0.82, referred to as RBD levels. RBD levels were replicated six times (chambers) for RBD of 0.67 and 0.72, seven times for RBD of 0.77, and five times for the highest tested level of 0.82. The unit of observation was not the chambers but the individual seedlings, which totaled 102 at the end of the 5.5-month-long experiment (n = 24 for RBD 0.67; n = 25 for RBD 0.72; n = 32 for RBD 0.77; n = 21 for RBD 0.82).
Equipment and instrumentation
A customized root growth-monitoring system (Soilinsight®) was used as a means to study the effects of varying RBD levels on seedling growth. The system was comprised of 24 chambers (internal dimensions of 30.6 cm in length, 8.2 cm in width, and 42.0 cm in height for a total volume of 10.5 L) with three sides constructed of stainless steel and the fourth side (face of chamber) constructed from 4-mm-thick glass to allow visualization of the soil and roots (Fig. 1a). Four frame systems were used to hold six chambers each (Fig. 1b). The frames allowed for a manual inclination of all chambers from 0° to 40°, and an inclination of 15° was selected for the study. The goal of inclining the chambers was to increase the likelihood of having a larger root mass growing on the side of the glass due to gravity. The entire system was placed in a laboratory in front of large-diameter windowpanes that were east facing. In addition to the relatively abundant natural sunlight, artificial grow lights (8 × 58 W, light output of 550 lumens, color rendering 70–79 Ra) mounted on a height adjustable assembly, were in operation 14 h per day during the entire test period. At soil contact, the combination of natural and artificial light provided a strength of 100 PAR (photosynthetic active radiation (µmol photons m−2 s−1)) as measured with a Li-189 light meter. To enable the movement of the soil-filled chambers (mass of 19–23 kg each), a 150 kg capacity electric winch was installed on a rail system mounted to the ceiling directly over the chambers. With the rail system and the winch, chambers could be raised from the racks and maneuvered in a safe manner.
Work procedure and description
Soil collection and compaction
Material required to fill the chambers was collected from a Norway spruce dominated forest near the town of Freising, Germany (48°24′37″N, 11°42′08″E). The litter layer was first removed, and then approx. 800 kg of soil (0–30 cm depth) was collected and transported to the Technical University of Munich for analysis. The soil was oven-dried at 60 °C for 48 h and then sieved through a 4.0-mm sieve with a mechanical sieve shaker. Following the initial preparation, the gravimetric moisture content of the soil was gradually increased to 16% to provide an adequate growing condition. At the bottom of every chamber, 550 g of sand was spread evenly and then a layer of fine gravel with a mass of 550 g was added on top of the sand layer (Fig. 2a). Both of these layers acted as a drainage system to ensure that the control valve located at the base of the chambers would not be clogged with soil. The last step prior to compaction was the addition of a semi-permeable fabric placed on top of the fine gravel to prevent the soil from mixing with the drainage layer. Within a chamber, the amount of soil needed to reach a target RBD level was added in three separate layers. Each layer was created using the same mass of soil poured from a fixed height of 20 cm with the use of a funnel. The amount of soil (wet basis) required for each lift was calculated based on the target RBD levels that considered the maximum bulk density (1.611 g cm−3) achieved through the standard Proctor tests performed on the same material. Prior to the compaction phase, a 10-mm-thick acrylic glass was placed in front of the glass side of the chamber and fixed with c-clamps for safety (Fig. 2b). The backside of the chamber was also supported with 19 mm plywood to prevent the deformation of the chamber during the compaction phase. Once the required mass of soil was added for the first layer (bottom of the chamber), a hydraulic press was used to compact the soil within the chambers by applying a vertical load on a loading plate that was built to fit inside the chambers and allow for uniform pressure distribution (Fig. 2c). Considering the height of the chambers, the thickness of individual compacted lifts was set at 12.3 cm. The process was repeated for the two additional layers. Once the three layers of 12.3 cm were completed, the chamber was placed in its respective frame and covered with plastic to limit moisture variation.
a Semi-permeable fabric placed on a layer of fine gravel placed on top of a layer of sand to create the needed drainage layer, b chamber placed on a 20-ton vertical hydraulic press, c example of compacted soil with top plate resting at the surface, d completely filled and compacted chamber ready for seeding
Because all sides of the Soilinsight chambers were fixed and could not be disassembled to allow control measurements, we constructed an exact replica (test chamber of same dimensions) from structural lumber. The test chamber had a removable front panel composed of 10-mm-thick acrylic glass to allow for soil sampling once the compaction process was completed. With the test chamber, the same compaction process as described above was performed for each RBD level using the same soil that was used within the Soilinsight chambers. Once a target RBD was reached with the hydraulic press, two soil cores with a volume of 100 cm3 were collected from each of the three layers for a total of six samples per RBD level. Soil samples were used to perform the analyses described in the section soil properties.
Seeding and extraction
Five spruce seeds (region of provenance: Alpine forest land 840 27) were sown per chamber on April 9, 2018 by creating a 8 mm deep furrow along the middle of the longitudinal axis of a chamber. Within a chamber, seeds were sown 5 cm apart and the material displaced to create the needed indent was carefully backfilled after sowing. On day 26 (after sowing), 82 of the 120 seeds germinated, thus providing a respectable 68% germination rate. However, to be able to investigate as many seedlings as possible and because of the lengthy and complex soil preparation process, the remaining 38 non-germinated seeds were replaced with germinated seeds of the same age (26 days old) that were sown as backup. Replanting germinated seeds in areas of deficit was done with the use of a cone-shaped instrument to create the small indentations needed within the soil to allow the positioning of the germinated seed. After the replanting phase, the tiny holes were carefully backfilled. To avoid the establishment of competing vegetation, a thin layer of sand (100 g per chamber) was uniformly added on the surface of each chamber and surrounded each seedling. For ease of identification and monitoring, every seedling obtained a tree identification code (TIDC). The TIDC included the RBD level, the number of the chamber (1–24), and the position of a seedling within the chamber (1–5). To differentiate replanted seedlings to those that were originally seeded, an additional letter was added where s referred to seeded and r referred to replanted. During the growing period, every chamber was subjected to a fixed water regime, which consisted of three irrigation events per week with the same volume of water provided to each chamber. To limit the disturbance of the soil, chambers were weighed ten times during the growing period and mass of the chamber was used as a proxy for soil moisture content within each RBD level.
The seedlings were extracted from the chambers 156 days after sowing to perform detailed analysis. For the extraction, the soil needed to be saturated with water for a 24-h period after which the seedlings were carefully rinsed out of the soil to maintain the integrity of the root systems (Baier 2005).
Measurements and analyses
Above- and belowground biomass
After extraction, two perpendicular measurements (0.1 mm accuracy) of every root collar were taken with a digital caliper. Individual seedlings were then placed on a white sheet of paper with a millimeter scale, and pictures were taken as reference. The total fresh mass of each seedling was determined with a digital scale (0.001 g accuracy). The number of branches per seedling was also noted to see how the seedling structure developed. Seedlings were then cut at the root collar with pruning shears, thus creating two main components (above- and belowground biomass). Individual root systems were placed on paper towels and sprayed with 70% ethanol, wrapped with paper towels, placed inside a plastic bag, sealed, and then stored in a refrigerator set at 5 °C to conserve the roots for upcoming analysis. The length of the shoot was measured from the cut point (former root collar) to the apical bud and corresponded to the height of the seedling.
Aboveground biomass was then oven-dried at 60 °C for 12 h and weighed. The weight of the stem with and without needles and the weight of the needles were recorded. In a further step, 33 needles were counted per seedling to compute the mass of 100 needles. This approach is commonly used when the mass of needles is not sufficient.
To analyze root properties, the WinRHIZO® 2019 software from Regent Instruments Inc. (Quebec, Canada) in combination with a high-resolution scanner (set at 600 dpi) were used. The image analysis system allowed assessing both the architecture (tips and forks) and the morphology (length of roots at eight different root diameter classes) of the roots (Table 1).
To gain further insight into the quality of the root system, the degree of mycorrhization was determined. The biocenosis between a tree and fungi is very important for the tree. Since the hypha of a fungi is considerably smaller in diameter than root hairs, it can reach water in pores which is normally inaccessible by roots (Matyssek et al. 2010). They are also crucial in the absorption of relatively immobile elements such as phosphorous (Kozlowski et al. 1991). The degree of mycorrhization was determined by following the methods developed by Shaanxi (2010). In this regard, a petri dish was prepared with a rectangular grid of lines (spaced by 2 cm) drawn on the bottom. Roots were cut into pieces of two centimeters in length and randomly placed on the glass, while making sure that root pieces did not overlap (Fig. 3a). Every horizontal and vertical line was numbered. With a stereomicroscope, any root segment crossing a line was observed to verify whether ectomycorrhiza or a single hypha was present (Fig. 3b) and the number of pieces with and without fungi was tallied. A quotient of pieces with mycorrhization and pieces without was computed for every seedling, thus providing an estimate of how many roots were in contact with mycorrhiza. In this study, no differentiation between short, middle, and long distance type of mycorrhiza was performed.
After the roots were analyzed with WinRHIZO® and the degree of mycorrhization was determined, they were oven-dried at 60 °C for 12 h according to Baier et al. (2006) and dry root mass was measured. Determining biomass values was important to compare different seedlings in terms of biomass allocation and accumulation. Therefore, the shoot–root ratio (SRR) (shoot with needles [g]/root [g]) and the root–leaf biomass ratio (RLB) (root [g]/needles [g]) were computed. The RLB identifies where biomass is allocated in order to increase capturing limiting external resources (Shipley and Meziane 2002). In addition, the specific root length (SRL) (m/g) was used as an indicator to assess cost and benefits of roots (Ostonen et al. 2007).
Nutrient uptake
To obtain information of the nutrient uptake of the seedlings, needles and roots were analyzed individually. The required dry mass of 30 mg per sample in combination with the rather light seedlings triggered the need to perform this particular analysis at a chamber level rather than at the seedling level. Therefore, 24 samples of needles and 24 samples of roots were analyzed. Additionally, one soil sample of every RBD level was analyzed to assess whether nutrient supply was sufficient for the growth of Norway spruce seedlings.
Prior to analysis, samples of needles, roots, and soil needed to be oven-dried at a maximum of 60 °C (Baier et al. 2006). Afterward, needles and roots were milled separately using an oscillating mill for a duration of 150 s at a frequency of 20 Hz. To assess the nutrient content, a mass spectrometry (inductively coupled plasma (ICP)) analysis focusing on key major elements (P, K, Ca, Mg, S, Fe, Mn, Cu, Zn, B) was performed as well as the determination of the cation exchange capacity (CEC expressed in cmolc kg−1) and percent of base saturation (BS). In addition, the C and N contents for the needles, roots and soil were also determined by a vario EL III elemental analyzer. Percent BS is the percentage of the CEC occupied by the basic cations Ca2+, Mg2+, and K+ as is determined by Eq. 2:
Soil properties
Since the experiment tested the response of Norway spruce seedlings to varying RBD levels, it was imperative to first determine the maximum bulk density achieved by performing a standard Proctor test according to ASTM D698 (12e2). This was done by taking a representative sample of the material used for testing with the Soilinsight system. Grain size distribution for grains larger than 0.063 mm was determined with sieves of varying apertures, while the size and proportion of smaller diameter grains were determined using the sedimentation process. The liquid and plastic limits were determined according to ASTM D4318 (17e1), while soil texture was determined based on the USDA texture triangle. The six soil cores collected from each RBD level, using the test chamber, were used to determine saturated hydraulic conductivity (kf) using an ICW Eijkelkamp permeameter (Eq. 3). The volume of coarse pores was determined with a pF unit from Eijkelkamp operated at 60 and 300 mbar. Lastly, the soil samples were oven-dried at 105 °C until constant mass was reached to obtained the soil dry bulk density.
where kf = permeability factor of a soil (m d−1), Q = quantity of through-flowing water (ml), L = length of sample (cm), H = level difference inside/outside the ring holder (cm), A = cross-sectional area of the sample (cm2), T = time it takes that Q flows through the sample (min), factor 144 = 144 (ml/cm2 min) = m d−1.
Statistical analyses
The main goal was to determine whether there were statistical differences between the independent variables (root mass, shoot mass, needle mass, root length, shoot length, needled length, etc.) and the treatments (RBD levels 0.67, 0.72, 0.77, 0.82). Agreement with the parametric assumptions was verified through the visual observation of the normal distribution of data and residuals and statistically with the use of the Shapiro–Wilk test and the Kolmogorov–Smirnov test. In case of parametric data, a one-way analysis of variance (ANOVA) was performed and differences were isolated to the different treatments (RBD levels) using the Tukey–Kramer’s HSD technique. When the data was nonparametric, logarithmic and exponential transformations were performed and normal distribution was tested again. If the data remained suited for nonparametric tests, a Kruskal–Wallis test was conducted to see whether there were statistical differences between the treatments and the Bonferroni correction was applied in the pairwise comparisons to verify which treatments were statistically different from one another. Pearson’s correlation between RBD levels and seedling parameters were also performed. The analyses were conducted using SPSS version 21 and Minitab version 18. A significance level of a < 0.05 was applied in all analyses.
Results
Soil physical properties and nutrient content
Soil collected from the forest and used for testing was classified as a silt loam according to the USDA soil classification. Grain size distribution and results of the consistency limits are presented in Table 2. The soil moisture and density relationship, expressed through the standard Proctor test, showed a maximum soil dry density of 1.611 g cm−3 (dry unit weight of 15.80 kN m−3) achieved at an optimum moisture content of 18.9% (Fig. 4). From this maximum dry density, the following dry densities 1.08, 1.16, 1.27, and 1.32 g cm−3 were targeted for RBD levels of 0.67, 0.72, 0.77, and 0.82, respectively. With the use of the test chamber, each RBD level was tested individually and six soil cores were collected per test. Mean values of soil permeability (kf) decreased with increasing RBD levels and were 1.11, 0.36, 0.20, and 0.11 m d−1 for the 0.67, 0.72, 0.77, and 0.82 RBD levels, respectively.
Soil moisture was estimated based on the weight of the chambers recorded over the test period, with a higher frequency of measurements occurring during the second half of the experiment. Because the mass of the seedlings was very low, a change in the mass of the chambers was mostly caused by variation in soil moisture. At the first weighing on day 68, soil moisture content ranged from 25.4 to 29.4% for RBD levels of 0.82 and 0.67, respectively (Fig. 5). Due to the rigid irrigation schedule, where all chambers received the same amount of water, the trends illustrated in Fig. 5 are similar across all RBD levels. To some extent, the overall tendency of soil moisture content seem to be linked to changes in ambient temperature (presented in Fig. 5) recorded in the laboratory where the experiment was held. An increase in ambient temperature was often in line with a decrease in average soil moisture content.
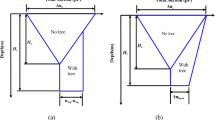
The results of the pore volume analysis showed a decrease of fast draining coarse pores as the tested RBD level increased, thus resulting in less air supply for roots to grow (Fig. 6). The volume of slow draining coarse pores was lowest at RBD 0.77 and was similar between the other RBD levels. The residual pore volume at 0.77 and 0.82 was higher than at RBD 0.67 and 0.72. It is important to note that the residual pore volume also contains fine pores that are too small to provide water for plants. The total pore volume ranged from a high of 60.4% to a low of 56.6% for RBD levels of 0.67 and 0.82, respectively.
In order to know which nutrients were provided by the soil to the plants, an ICP analysis as well as base saturation and cation exchange capacity was conducted on one soil sample per RBD level. The values for CEC increased from 141.5 to 145.4 for RBD levels of 0.67 and RBD 0.82, while the percent of base saturation was similar for all tested RBD levels (Table 3).
Besides CEC and BS, the nutrient composition of the soil regarding the most important elements was analyzed and compared to values analyzed within the needles and roots. Table 4 provides an overview of nutrient content of the soil. On the left side of the table, nutrients are presented in mg g−1 and on the right side in µg g−1. This division enables the comparison with nutrient contents within needles and roots.
Biomass properties and nutrient content
When comparing the height of the tallest seedling per chamber across the four RBD levels, very similar responses were observed for the first 60 days after sowing (Fig. 7). Variations in seedling height began to occur at day 60 for the RBD of 0.77 and at day 95 for the RBD of 0.82, where the two higher RBD levels demonstrated lower vertical growth rates as compared to the other two lighter RBD levels. As time progressed, the seedlings growing in soil at RBD level of 0.72 also experienced reduced vertical growth rates. At the end of the monitoring period, height of the tallest seedling per chamber was negatively correlated with RBD level.
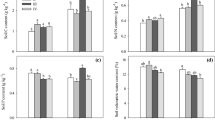
After extraction, individual seedling components (root, shoots, and needles) were oven-dried and weighed. Irrespective of seedling component, a clear trend of decreasing dry mass per seedling was observed as RBD levels increased from 0.67 to 0.82 (Fig. 8). Average root dry mass per seedling ranged from 56 mg (RBD 0.67) to 29 mg (RBD 0.82), thus corresponding to a 48% lower average mass for seedlings growing on soils at RBD 0.82 as compared to those exposed to the RBD level of 0.67 (Fig. 8a). Based on a Kruskal–Wallis test, a statistical difference (p = 0.010) in average root dry mass per seedling was found between RBD levels. The Bonferroni pairwise comparison showed that the average root dry mass was statistically lower between the 0.67 and the 0.77 and 0.82 RBD levels. Similarly, statistically higher (p = 0.002) shoot dry mass per seedling was recorded at RBD 0.67 as compared to RBD 0.82 (Fig. 8b). Within a respective RBD level, the needles corresponded to the highest dry mass compared to the other tested seedling components (Fig. 8c). The average dry mass of needles per seedling ranged from 77 to 44 mg for the RBD levels of 0.67 and 0.82, respectively, thus equaling a 43% difference in mass between the two RBD levels. A statistical difference (p = 0.010) was detected between the lowest and the highest RBD levels.
Biomass allocation within a seedling was analyzed by comparing root length, root collar diameter and shoot height (Table 5). For visualization, a representative seedling from each RBD level is presented to scale in Fig. 9. Average root length per seedling varied from 65.2 to 30.4 cm for the RBD levels of 0.67 and 0.82 (53.3% reduction compared to the RBD of 0.67), respectively, with statistical differences detected between the two groups based on a Kruskal–Wallis test followed by a Bonferroni pairwise comparison. Variation in root collar diameters between RBD levels was less than for root length, and no statistical differences were detected between the RBD levels. Root collar diameter ranged from 1.52 to 1.35 mm for RBD levels of 0.67 and 0.82, respectively. Very similar trends were detected for shoot length, where despite showing a 19% reduction between the 0.67 and 0.82 RBD levels, no statistical differences were found.
To gain additional insight on belowground root biomass, root length was analyzed and presented by diameter classes (Fig. 10). A trend of decreasing root length as RBD level increased was observed for the three smallest root diameter classes (< 0.10 mm, 0.10–0.20 mm, and 0.21–0.30 mm) with statistical differences between the two tested RBD extremes of 0.67 and 0.82 as well as between the RBD levels of 0.67 and 0.77 based on Kruskal–Wallis test followed by a Bonferroni method. Root length for the 0.31–0.40 mm and 0.41–0.50 mm diameter classes showed less variation between RBD levels, and no statistical differences were detected across RBD levels. When focusing on the larger diameter roots (0.51–1.00 mm), statistical differences in average root length per seedling were observed between RBD 0.67 and RBD of 0.77 as well as RBD 0.82.
Average root length per seedling per root diameter class and RBD level. Circles represent the mean and different letters between treatments (RBD levels) indicate a statistical difference based on pairwise comparison using the Kruskal–Wallis test followed by the Bonferroni method at a 0.05 probability level
The complexity of the root system and its architecture can be explained in part by the assessment of the number of root tips and forks. Similar to previous findings, both the number of root tips and root forks per seedling decreased with increasing RBD level and the most pronounced difference was detected between the RBD of 0.67 and 0.72. Continued increase in RBD beyond 0.72 caused fewer tips and roots per seedling but to a lower extent. Number of root tips per seedling decreased from 244 to 122 for RBD levels of 0.67 and 0.82, respectively, with statistical differences between means from both RBD levels according to a Bonferroni method following a Kruskal–Wallis test (Fig. 11a). The number of root tips per seedling was far greater for the RBD of 0.67 as compared to all other tested RBD levels. A similar trend can be observed for the number of root forks per seedling (Fig. 11b). In this regard, a difference of 56% was recorded between RBD levels of 0.67 (302 root forks per seedling) and 0.82 (132 root forks per seedling), with statistical differences detected between both groups.
To complete the growth assessment, Pearson’s correlation coefficients were determined between RBD levels and seedling parameters (Table 6). Most of the seedling parameters tested were at least significant at the probability level of 0.05 and the only parameters that were not statistically significant were root collar diameter and the length of root with diameters ranging from > 0.4–0.5 mm and > 1.0 mm.
Nutrient composition for roots and needles is presented in Table 7. As the RBD levels increased from 0.67 to 0.82, the concentration of P, K, and Mg in the root system decreased by 15%, 28%, and 16%, respectively, whereas the concentration of N did not show this decreasing trend. In addition to providing the nutrient contents of the needles, it was also of interest to understand how these numbers were in relation to the needed nutrients of Norway spruce. In general, the content of P was too low over all RBD levels for the seedlings to grow at their full potential, while the level of iron was too high across all RBD levels. Additionally, the high level of Ca within the seedling needles could place them at risk of calcium chlorosis. All other values were in a lower normal range to luxury surplus. A decrease of specific elements with increasing RBD level, as was the case with the root systems, could not be detected with the needles.
Growth ratios
The computed growth ratios (SRR, SRL, and RLB) showed no statistical differences between the tested RBD levels (Table 8). At RBD 0.72, one seedling needed to be removed for the SRR analysis and two for the SRL analysis because of being extreme outliers. Mean SRR varied from 2.34 to 2.98 for RBD levels of 0.67 and 0.72, respectively. To get a more detailed view, the RLB ratio was computed and showed that more biomass was accumulated within photosynthetic active biomass as compared to roots. The mean RLB ratios increased from 0.74 to 0.82 for the RBD levels of 0.77 and 0.67, respectively.
Degree of mycorrhization
The degree of mycorrhization can provide an indication on the probability of seedling survival. Overall, 62 seedlings showed a colonization with fungi and since five seedlings from the RBD 0.72 and one seedling from the RBD 0.77 could not be analyzed because their root systems were too dry, an overall mycorrhization of 64.6% was observed (62 out of 96 seedlings). The degree of mycorrhization ranged from 0.07 to 0.17 for RBD levels of 0.82 and 0.67, respectively (Table 9). However, based on a Kruskal–Wallis test, no statistical difference was reported between the different RBD levels. As an example, a black hypha of a fungi connected to a root segment of approx. 1.5 mm diameter can be seen (identified with the arrow) in Fig. 12.
Discussion
Relative bulk density as a measure for compaction
Relative bulk density is determined as the ratio of field bulk density and a site-specific bulk density, normally referred to as maximum bulk density (Stengel et al. 1984). In our experiment, the reference bulk density for the soil used for testing was determined by the standard Proctor test, which provided a maximum bulk density (1.611 g cm−3) at the optimum moisture content (18.9%). Since we used the same soil for both the standard Proctor tests and as substrate in the chambers of the Soilinsight system, results can be compared to other studies that used the RBD method. This would not have been the case if only absolute bulk density (g cm−3) would have been used since it is strongly influenced by soil texture, organic matter content, and other soil properties. RBD values of 0.80 and higher have been linked to limited values of water availability, soil mechanical resistance, and air-filled porosity, thus ultimately identifying soil with degraded physical conditions that can impede the growth of certain agricultural crops and forested tree species (da Silva et al. 1994; Bulmer and Simpson 2005; Labelle and Jaeger 2011; Labelle et al. 2019). Our results are in line with findings from Zhao et al. (2010) and Kormanek et al. (2015) who reported reduced growth of Douglas fir and sessile oak (Quercus patrae Liebl.) seedlings when exposed to RBD levels exceeding 0.80 or 80% of the maximum bulk density, as determined by the standard Proctor test. Using RBD as a measure for compaction and associated growth impediments for Norway spruce was innovative and provided a repeatable method to assess the quality of soil properties. The importance of Norway spruce on German public forests is paramount, in terms of both forested area (43%) and economic output through wood volume sales (66%). These first laboratory results can be a foundation for future work that would link, which operational parameters (machine axle loads, traffic frequency, dynamic ground pressures, etc.) need to be controlled and under which terrain conditions (topography, organic matter content, soil moisture content range, etc.) in order to minimize the area of a harvest site subjected to high growth-impeding RBD levels.
Growth-limiting factors
Despite controlling both the frequency and volume of water provided to each chamber, differences in average soil moisture content per RBD level seem to indicate that other factors were at play. As the RBD of a chamber increased, the amount of time needed for the water to infiltrate the soil surface and then percolate through the chamber was much higher as compared to the time needed for the water to seep through the chambers with lower RBD levels. The high ambient temperature in combination with the slow infiltration rate observed in chambers exposed to a higher RBD could have allowed a higher share of evaporation to occur, thus affecting the moisture content of the soil.
With a CEC value over 140, the exchange capacity of the soil used for testing was high and could be problematic for the long-term growth of Norway spruce. A high CEC in combination with an annual mean temperature over 7.5 °C or higher can greatly increase the risk of red rot (Arbeitskreis Standortskartierung 2016). The base saturation with values of 98% was also extremely high. Most of the base saturation was caused by the significant amount of Ca, which can lead to calcium chlorosis should the experiment have lasted for several years (Hartmann et al. 1995). Values of SRL in our study were about half of those reported by Konofsky (2018) for 2-year-old Norway spruce seedlings grown under normal conditions.
Substrate material used for testing was collected from a local Norway spruce dominated forest. However, despite this condition, results for nutrient supply were heterogeneous. Concentration of elements such as Ca and Mg was too high, whereas the seedlings probably received lower amounts of P. The nutrient analysis of the soil for the different RBD levels was performed at the end of the growth experiment. Those results can indicate if compaction had an influence on nutrient composition of the soil, which was not the case.
Unlike the needles, roots showed a decrease of N, P, K, and Mg with increasing RBD but differences were not statistically significant. The fact that needles showed no differences was possibly caused by the short duration of the experiment. In future studies it needs to be investigated if compaction has an influence on nutrient composition of roots and needles. If a specific nutrient composition of tree compartments compared to the nutrient supply by the soil could be linked to a specific level of compaction this would help to identify growth-impeding compaction more easily.
The interpretation of the needle level values derived from the Norway spruce seedlings has to be done carefully. Fully valid results can only be delivered by needles taken from the trees between October and February. Until hibernation, the nutrient composition within the needles is changing (Gutachterausschuss Forstliche Analytik 2005). This means that the nutrient contents of the needles reported in this study are only snapshots. A closer look on the needle level values shows that there is no difference in nutrient uptake at different RBD levels. To obtain more specific values, a larger sample size should be investigated and seedlings should not be extracted before sufficient biomass is available for analysis (30 mg per sample).
Shoot and root structure
The measurements done to assess the shoot structure showed now statistical difference between the RBD levels despite showing a trend of decreasing height development as RBD levels increased. Over the 156-day growth-monitoring period, the height of all Norway spruce seedlings were 20% higher for the RBD 0.67 (6.1 cm) as compared to the RBD 0.82 (4.9 cm). In a study performed by Kormanek et al. (2015), average shoot height of sessile oak seedlings ranged from 15.2 to 16.4 cm after a 76-day experiment for RBD levels of 0.85 (1.276 g cm−3) and 0.52 (0.808 g cm−3), respectively. Findings from Kormanek et al. (2015) show that a fast response of height development is probably highly dependent on tree species. Nevertheless, seedling height should continue to be measured because it provides a nondestructive assessment that can be recorded quickly over the entire length of an experiment. It would have been interesting to prolong the study to detect whether the decreasing height trend, as observed for seedlings exposed to the highest RBD level of 0.82 from day 140 onwards, would be maintained (Fig. 7).
We observed that root collar diameter decreased by 11% between the RBD of 0.67 and 0.82. Similar to findings from Kormanek et al. (2015), no statistical differences in root collar diameters were detected between RBD groups. Because the percent difference in shoot height was more pronounced between the two RBD extremes of 0.67 and 0.82 than for the root collar diameter, it is possible that the seedlings allocated more energy to height growth as opposed to diameter growth. When considering aboveground architecture, seedlings at RBD 0.67 had on average one branch whereas no branches were present on seedlings from the other RBD levels. When all those values are considered, a clear trend of decreasing shoot structure was observed as compaction was increased. In certain instances, it was more difficult to identify the exact location of the root collar and because of this, potential variations in root collar diameters could also be explained in part by slight differences in measurement location.
Values for root structure were more significant than for shoot structure. The number of tips as well as the number of forks per seedling decreased with increasing RBD. The root tips are important particularly for water uptake and for root growth (Roloff 2013), whereas the number of forks provides an indication of the complexity of the root system. Furthermore, the length of roots within different root diameter classes was significantly higher at RBD 0.67 than within the other RBD levels. Forks and the root length in RDC show that the root system of seedlings grown at RBD 0.67 was more complex than the roots of other seedlings. The sum of root length showed a decreasing trend with increasing RBD level. The combination of the results from the RDCs and from the total root length showed that the roots decreased in diameter and length as the RBD level increased. Our findings are in line with results from Siegel-Issem et al. (2005) and Kormanek et al. (2015) who observed a negative change of root length as soil became more compacted. The values for root structure showed that seedlings grown at lower RBD levels had an advantage in nutrient and water uptake and were better anchored in the soil because of a longer and denser root system. This advantage resulted in higher biomass accumulation when soil was less compacted. Longer-term trials would shed some light to see whether these advantages have compound effects on biomass growth.
When focusing on the growth ratios, the mean SRR values ranged 2.34 to 2.98 for RBD levels of 0.67 and 0.72, which is in the normal range (1 to 3) as reported by Stimm et al. (2013) and indicates that more biomass is accumulated above ground in the form of shoot and needles as compared to the belowground biomass.
Mycorrhization
Although comparative information for the grade of mycorrhization of Norway spruce seedlings at this early age is lacking in published literature, the values reported seemed to be rather low. Baier (2005) reported that 4-year-old Norway spruce seedlings in the Bavarian limestone Alps had a grade of mycorrhization between 0.5 to 1.0, considerably higher than the range of 0.07 and 0.17 reported in our study for RBD levels of 0.82 and 0.67, respectively. It is also possible that the time elapsed between the seedling extraction and the mycorrhization analysis was too long. Despite taking precautions by keeping the root systems stored at a constant temperature of 5 °C, the analysis could not be performed in the period of 3 days as suggested by Baier (2005). Future studies should consider performing the mycorrhization analysis soon after the extraction of the seedlings and then proceed to the more detailed root analysis. Although the roots needed to be segmented into pieces for analyzing the mycorrhiza, a software such as WinRHIZO has functions to allow the linking of root parts, thus possibly still providing meaningful results (Bauhaus and Messier 1999). If storage for several days is needed or likely to happen, it is also possible to place the roots into sealable containers filled with a mixture of alcohol and water. This method delivered valid results for mycorrhization even several weeks after the extraction (Zehle 2018).
Study limitations and recommendations
This was one of the first studies using the Soilinsight system that focused on seedlings. In general, the system performed well, particularly after modifications were done and the compaction process was tailored for the experiment. The winch system installed over the chambers proved to be extremely valuable and allowed for safe maneuvering of the chambers. The individual chambers were of adequate size to allow the growth of five seedlings per chamber over the 156-day test period. It is possible that space within the chamber could become a limiting factor if the monitoring period was greatly extended or if additional seedlings were monitored per chamber. Despite performing weekly scans of each Soilinsight chambers, the root mass growing in contact with the glass side of the chambers was not sufficient across all RBD levels to perform a detailed analysis. Prolonging the monitoring period should alleviate this problem and then allow another method to quantify the effects of RBD levels on root growth, while considering the temporal scale. Another option could be to incline the chambers beyond the 15 degrees that was used in this study, thus increasing the likelihood of having a higher root mass growing near the glass. However, increasing the inclination of the chambers might cause the seedlings to grow even more lopsidedly, which could add further stress on the root collar, thus possibly influencing seedling growth.
Soil preparation, including the compaction process, was a very time-consuming task that required the researchers to follow a precise methodology to assure that all chambers were compacted uniformly to the target RBD levels. Because of this, and the mortality rate of the Norway spruce seedlings of 32%, we decided to replant about one-third of the seeds that had not germinated. Despite using germinated seeds that were sown on the same day of those sown in the chambers, it is possible that the replanting caused some stress to the germinated seeds, which could have impacted their growth behavior, particularly since the experiment was not extended over several years. During the replanting phase, special care was given to limit the exposure of the root to sunlight and replant as quick and as carefully as possible. Our analysis did not show any statistical differences between original seedlings to those that were replanted. However, it is recommended that future studies limit the frequency of replanting or attempt to increase original sample size to account for mortality.
Root analysis with WinRHIZO was rather straightforward. It is important to mention that the roots scanned during the experiment were of rather small dimensions due to the limited growing period. Complications in scanning more developed and complex root systems could arise when extending the growth-monitoring period or when studying tree species with more dense root systems. In these instances, additional care should be given to adequate root placement on the glass of the scanner in order to limit the frequency of root overlaps.
Conclusion
This laboratory study assessed the above- and belowground growth response of Norway spruce (Picea abies [L.] Karst) seedlings exposed to varying levels of relative bulk density (RBD), defined as the ratio between field bulk density and maximum bulk density, as determined by the standard Proctor test. With a customized system comprised of 24 individual chambers, soil collected from the forest was uniformly compacted to different RBD levels (0.67, 0.72, 0.77, and 0.82) in three equal layers and the growth of seedlings was monitored for 156 days. Statistically lower root (48%), shoot (43%), and needle (43%) mass per seedling for seedlings growing on the RBD 0.82 as compared to those growing on the RBD 0.67 were reported, thus indicating a clear trend of negative growth impact as soil compaction increases. In general, the two lowest RBD levels revealed no statistical differences, whereas statistical differences were observed as the RBD levels increased to 0.77 and 0.82. While considering the importance of Norway spruce within the German forestry sector and the increased rate of mechanized forest operations, results from the study highlight the need for additional research into the complex machine/soil/plant interactions. The use of RBD as a qualitative assessment of soil properties was supported. With significant growth reductions found for RBD levels of 0.77 and above, special care should be given to forest operations performed on sensitive sites or on soils with preexisting high soil densities to try and limit the extent of soils increasing beyond the problematic RBD levels and thus minimize the negative impact on biomass growth of Norway spruce seedlings.
References
Arbeitskreis Standortskartierung (2016) Forstliche Standortsaufnahme: Begriffe, Definitionen, Einteilungen, Kennzeichnungen, Erläuterungen. 7. Auflage. Eching bei München: IHW-Verlag. ISBN: 9783930167807
ASTM D698 12e2 (2012) Test method for laboratory compaction characteristics of soil using standard effort (12,400 ft-lbf/ft3 (600 kN-m/m3)). ASTM International, West Conshohocken
ASTM D4318 17e1 (2017) Standard test methods for liquid limit, plastic limit, and plasticity index of soils. ASTM International, West Conshohocken
Baier R (2005) Wurzelentwicklung, Ernährung, Mykorrhizierung und “positive Kleinstandorte” der Fichtenverjüngung (Picea abies [L.] Karst.) auf Schutzwaldstandorten der Bayerischen Kalkalpen. “Root development, nutrition, mycorrhization and “positive small sites” of spruce regeneration on protective forest sites of the Bavarian alps”. Ph.D. Dissertation. Technische Universität München, Freising, Germany. https://opac.ub.tum.de/search?bvnr=BV021511263
Baier R, Ettl R, Hahn C, Göttlein A (2006) Early development and nutrition of Norway spruce (Picea abies (L.) Karst.) seedlings on different seedbeds in the Bavarian limestone Apls—a bioassay. Ann For Sci 63:339–348
Bauhaus J, Messier C (1999) Evaluation of fine root length and diameter measurements obtained using RHIZO image analysis. Agron J 91:142–147
Bayerische Staatsforsten AöR (2018) 2018: Bayerische Staatsforsten Statistikband [2018: Bavarian State Forest Statistics book]. Regensburg, Germany, p 30
Binkley D, Fisher RF (2013) Ecology and management of forest soils, 4th edn. Wiley, New York, p 347
Blackwell PS, Graham JP, Armstrong JV, Ward MA, Howse KR, Dawson CJ, Butler AR (1986) Compaction of a silt loam soil by wheeled agricultural vehicles. I. Effects upon soil conditions. Soil Tillage Res 7:97–116
Brady NC, Weil RR (1996) The nature and properties of soils, Eleventh edn. Prentice Hall, Upper Saddle River, p 740
Bulmer CE, Simpson DG (2005) Soil compaction and water content as factors affecting the growth of lodgepole pine seedlings on sandy clay loam soil. Can J Soil Sci 85(5):667–679. https://doi.org/10.4141/S04-055
Cambi M, Giannetti F, Bottalico F, Travaglini D, Nordfjell T, Chirici G, Marchi E (2018) Estimating machine impact on strip roads via close-range photogrammetry and soil parameters: a case study in central Italy. iForests 11:148–154
Carter MR (1990) Relative measures of soil bulk density to characterize compaction in tillage studies on fine sandy loams. Can J Soil Sci 70:425–433
Coder KD (2016) Soil compaction stress and trees: a workbook of symptoms, measures and treatment. Pub. No. 38. Warnell School of Forestry and Natural Resources. University of Georgia
Costello LR, MacDonald JD, Jacobs KA (1991) Soil aeration and tree health: correlating soil oxygen measurements with the decline of established oaks. USDA Forest Service General Technical Report. PSW-126
Craig RF (2005) Soil mechanics, 7th edn. Spon Press, British Library Cataloguing in Publication Data, London
da Silva AP, Kay BD, Perfect E (1994) Characterization of the least limiting water range of soils. Soil Sci Soc Am J 58(6):1775–1781
DIN 18127 (2012-09) Baugrund, Untersuchung von Bodenproben – Proctorversuch. Deutsches Institut für Normung e. V
Ezzati S, Najafi A, Rab MA, Zenner EK (2012) Recovery of soil bulk density, porosity and rutting from ground skidding over a 20-year period after timber harvesting in Iran. Silva Fenn 46:521–538
Froehlich HA, Miles DWR, Robins RW (1986) Growth of young Pinus ponderosa and Pinus concorta on compaction soils in Central Washington. For Ecol Manag 15:285–294
Fründ H-C, Averdiek A (2016) Soil aeration and soil water tension in skidding trails during three years after trafficking. For Ecol Manag 380:224–231
Giannetti F, Chirici G, Travaglini D, Bottalico F, Marchi E, Cambi M (2017) Assessment of soil disturbance caused by forest operations by means of portable laser scanner and soil physical parameters. Soil Sci Soc of Am J 81:1577–1585
Greacen EL, Sands R (1980) Compaction of forest soils: a review. Aust J Soil Res 18(2):163–189
Gutachterausschuss Forstliche Analytik (Hg.) (2005) Handbuch Forstliche Analytik. “Handbook for forestry analytics”. https://www.bmel.de/SharedDocs/Downloads/Landwirtschaft/Wald-Jagd/Bodenzustandserhebung/Handbuch/HandbuchForstanalytikKomplett.pdf?__blob=publicationFile. Accessed 14 Nov 2018
Håkansson I, Lipiec J (2000) A review of the usefulness of relative bulk density values in studies of soil structure and compaction. Soil Tillage Res 53(2):71–85. https://doi.org/10.1016/S0167-1987(99)00095-1
Han H-K, Han H-S, Page-Dumroese DS, Johnson L (2009) Soil compaction associated with cut-to-length and whole tree harvesting of coniferous forest. Can J For Res 39:976–989
Hartmann G, Nienhaus F, Butin H (1995) Farbatlas Waldschäden. Diagnose von Baumkrankheiten. 2., überarb. und erw. Aufl. Stuttgart: Ulmer. ISBN 9783800133512
Jourgholami M, Ghassemi T, Labelle ER (2019) Soil physio-chemical and biological indicators to evaluate the restoration of compacted soil following reforestation. Ecol Ind 101:102–110. https://doi.org/10.1016/j.ecolind.2019.01.009
Konofsky LM (2018) Analyse der Biomasseverteilung von Fichtensämlingen verschiedener Herkünfte aus Bayern. Bachelorarbeit. “Analysis of the biomass distribution of spruce seedlings of different provenances from Bavaria”. Technische Universität München, Freising, Germany
Kormanek M, Głąb T, Banach J, Szewczyk G (2015) Effects of soil bulk density on sessile oak Quercus petraea Liebl. seedlings. Eur J For Res 134(6):969–979
Kozlowski TT (1999) Soil compaction and growth of woody plants. Scand J For Res 14(6):596–619
Kozlowski TT, Kramer PJ, Pallardy SG (1991) The physiological ecology of woody plants. Hartcourt Brace Javanovich, Publishers, San Diego, p 655
Labelle ER, Jaeger D (2011) Soil compaction caused by cut-to-length forest operations and possible short-term natural rehabilitation of soil density. Soil Sci Soc Am J 75(6):2314–2329
Labelle ER, Jaeger D (2019) Management implications of using brush mats for soil protection on machine operating trails during mechanized cut-to-length forest operations. Forests 10(1):19
Labelle ER, Poltorak BJ, Jaeger D (2019) The role of brush mats in mitigating machine induced soil disturbances: an assessment using absolute and relative soil bulk density and penetration resistance. Can J For Res 49(2):164–178
Lipiec J, Håkansson I (2000) Influences of degree of compactness and matric water tension on some important plant growth factors. Soil Tillage Res 53:87–94
Matyssek R, Fromm J, Rennenberg H, Roloff A (2010) Biologie der Bäume. Von der Zelle zur globalen Ebene. Stuttgart: Verlag Eugen Ulmer (UTB Biologie, Agrar- und Forstwissenschaften, Landschaftsplanung, 8450)
Murphy G, Firth JG, Skinner MF (2004) Long-term impacts of forest harvesting related soil disturbance on log product yield and economic potential in a New Zealand forest. Silva Fenn 38:279–289
Naghdi R, Solgi A, Labelle ER, Zenner EK (2016) Influence of ground-based skidding on physical and chemical properties of forest soils and their effects on maple seedling growth. Eur J For Res 135(5):949–962. https://doi.org/10.1007/s10342-016-0986-3
Ostonen I, Püttsepp Ü, Biel C, Alberton O, Bakker MR, Lõhmus K (2007) Specific root length as an indicator of environmental change. Plant Biosyst Int J Deal All Asp Plant Biol 141(3):426–442. https://doi.org/10.1080/11263500701626069
Pidgeon JD, Soane BD (1977) Effects of tillage and direct drilling on soil properties during the growing season in a long-term barley mono-culture system. J Agric Sci 88(2):431–442
Poltorak BJ, Labelle ER, Jaeger D (2018) Soil displacement during ground-based mechanized forest operations using mixed-wood brush mats. Soil Tillage Res 179:96–104
Power WE (1974) Effects and observations of soil compaction in the Salem District. USDA Bureau of Land Management. Technical Note 256: 1–11
Rohand K, Al Kalb A, Herbauts J, Verbrugge JC (2004) Changes in some mechanical properties of a loamy soil under the influence of mechanized forest exploitation in a beech forest of central Belgium. J Terramech 40:235–253
Roloff A (2013) Baumpflege. 2. Aufl. Stuttgart-Hohenheim: Eugen Ulmer Verlag (Forstwirtschaft). ISNB 978-3-8001-7895-7
Schaefer M, Schauermann J (1990) The soil fauna of beech forests: comparison between mull and a moder soil. Pedobiologia 34(5):299–314
Shaanxi J (2010) Nutritional characterization of young trees in a protective forest as influenced by plant species, site and mycorrhization. Master thesis, Northwest A & F University, China. Phytochemistry
Shipley B, Meziane D (2002) The balanced-growth hypothesis and the allometry of leaf and root biomass allocation. Funct Ecol 16(3):326–331. https://doi.org/10.1046/j.1365-2435.2002.00626.x
Siegel-Issem CM, Burger JA, Powers RF, Ponder F, Patterson SC (2005) Seedling root growth as a function of soil density and water content. Soil Sci Soc Am J 69(1):215–225. https://doi.org/10.2136/sssaj2005.0215
Stengel P, Douglas JT, Guérif J, Goss MJ, Monnier G, Cannell RQ (1984) Factors influencing the variation of some properties of soils in relation to their suitability for direct drilling. Soil Tillage Res 4:35–53
Stimm B, Blaschke H, Rothkegel W, Ruppert O (2013) Stabilität hat tiefe Wurzeln. Die ideale Forstpflanz: Aspekte einer funktionalen Beziehung zwischen Wurzel und Spross. “Stability has deep roots. The ideal forest plant: aspects of a functional relationship between root and shoot”. LWF aktuell-Magazin der Bayerischen Landesnastalt für Wald und Forstwirtschaft und Mitgliederzeitschrift des Zentrum Wald-Forst-Holz Weihenstephan 20(93):9–14
Zehle R (2018) Durchwurzelungsintensität und Mykorrhizierung entlang von Standortsgradienten im Bayerischen Wald. “Root growth intensity and mycorrhyzation along site gradients in the Bavarian forest“. Master thesis, Technische Universität München, Freising, Germany
Zhao Y, Krzic M, Bulmer CE, Schmidt MG, Simard SW (2010) Relative bulk density as a measure of compaction and its influence on tree height. Can J For Res 40:1724–1734
Acknowledgements
This work was financially supported by the Assistant Professorship of Forest Operations at the Technical University of Munich. The authors wish to acknowledge the professional insight provided by Joachim Schattauer, Prof. Dr. Axel Göttlein, Dr. Lorenz Breinig, and Dr. Bernd Stimm all employed at the Technical University of Munich. Technical assistance provided by Michael Miesl and Marcel Labelle was truly appreciated.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Rüdiger Grote.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Labelle, E.R., Kammermeier, M. Above- and belowground growth response of Picea abies seedlings exposed to varying levels of soil relative bulk density. Eur J Forest Res 138, 705–722 (2019). https://doi.org/10.1007/s10342-019-01201-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10342-019-01201-6