Abstract
Zoophytophagous hemipteran predators provide relevant biological control services and their applications are consolidated in greenhouse pest management. The use of plant essential oils (EOs) for sustainable crop protection is being currently promoted. However, further knowledge of the potential side effects of EOs on predatory mirids (Hemiptera: Miridae) is required. Here, we evaluated the non-target impact of four EOs (anise, fennel, garlic and lavender) on the generalist predator Nesidiocoris tenuis (Reuter) in the laboratory. The baseline toxicity of EOs was firstly assessed on N. tenuis adults following topical contact exposure. Then, the predator reproduction and orientation behavior was tested following the exposure to three estimated EO lethal concentrations (LC1, LC10 and LC30). Garlic EO had the lowest estimated LCs (e.g., LC30 1.34 mg mL−1), being thus the most toxic compound among the tested EOs. The estimated LC30s for lavender, anise and fennel EOs were 2.75, 4.55 and 5.17 mg mL−1, respectively. The fertility and the orientation behavior of N. tenuis females was negatively affected by all the EOs at the highest tested concentration. Nevertheless, anise EO at LC1 and LC10 caused no sublethal effects on N. tenuis. Our findings suggest that careful attention should be given when EOs are used in combination with N. tenuis in pest management programs.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The use of zoophytophagous hemipteran predators is a consolidated practice for the biological control of agricultural pests (Thomine et al. 2020; Pérez‐Hedo et al. 2021a; van Lenteren et al. 2021). Also their role as plant defense elicitors has been recently exploited in several horticultural systems (Pérez‐Hedo et al. 2021b). Among predatory mirid bugs (Hemiptera: Miridae), Nesidiocoris tenuis (Reuter) is used for suppressing arthropod pest populations in Mediterranean protected crops because it can spontaneously inhabit various crops or easily purchased in bio-factories for augmentative releases (Castañé et al. 2011; Calvo et al. 2012; Zappalà et al. 2013; Naselli et al. 2017). The mirid predator has the capacity to be highly predaceous and the ability to survive on plants when prey is scarce, although plant damage can occur due to its herbivory occur at high population levels and increasing temperatures (Madbouni et al. 2017; Siscaro et al. 2019; Ingegno et al. 2021).
Albeit this controversial aspect, N. tenuis is routinely used in tomato crops as a biocontrol agent against leafminers, whiteflies and spider mites (Biondi et al. 2016). In fact, the introduction of the South American tomato pinworm, Tuta absoluta (Meyrick) (Lepidoptera: Gelechiidae), in the Mediterranean became one of the main purposes of N. tenuis releases in tomato crops, as this invasive pest can cause serious yield losses in tomato (Biondi et al. 2018; Desneux et al. 2021; Mansour and Biondi 2021).
Conventional management of arthropod pests often relies on repeated applications of synthetic pesticides which frequently have adverse effects on non-target organisms, such as physiological and behavioral (Desneux et al. 2007). As eco-friendly alternative, botanicals have been recognized as efficient pest control tools, with plant essential oils (EOs) being their most emphasized category (Regnault-Roger et al. 2012; Rathore 2017). EOs are secondary plant metabolites constituted by volatile and semi-volatile compounds involved in defense mechanisms against biotic and abiotic factors (Walling 2000; Hare 2011; Miresmailli and Isman 2014). With a low toxicity toward mammalians and negligible persistence in the environment, EOs have been increasingly suggested for application in organic and IPM programs (Campolo et al. 2017; Giunti et al. 2019; Pavela et al. 2020).
Despite their potential benefits, EOs have constitutive drawbacks linked to their properties, such as phytotoxicity, low solubility in water, high volatility and fast degradation (Moretti et al. 2002; Regnault-Roger et al. 2012; Krzyżowski et al. 2020). Nevertheless, recent advances in nanotechnology can remedy these inconveniences through a constant release of the active ingredients, a reduction in degradation, and by improving their solubility and stability patterns (Kah et al. 2013; de Oliveira et al. 2014; Campolo et al. 2017, 2020a; Maroofpour et al. 2021; Cherif et al. 2022).
The integration between EOs and the predator N. tenuis can be an interesting strategy to improve the effective management of arthropod greenhouse pests, such as T. absoluta. However, the insecticidal activity of EOs might be also harmful toward N. tenuis by causing lethal and sublethal effects, such as the reduction in population growth parameters and biological control capacity (Soares et al. 2019). In fact, EOs have often broad-spectrum activity, resulting in direct insect mortality or side effects, such as antifeedant and/or repellent behavior, oviposition deterrence and growth regulation, both to pests and beneficial organisms, such as pollinators and biological control agents (Isman and Tak 2017; Rathore 2017; Kim et al. 2021).
Although numerous studies have focused on the EOs bioactivity toward greenhouse insect pests (Campolo et al. 2017; Dunan et al. 2021; Sciortino et al. 2021; Tortorici et al. 2022), limited knowledge on EOs non-target effect toward beneficial arthropods has been produced. Earlier laboratory studies assessed the non-target impact of Citrus spp., Mentha pulegium L. and garlic (Allium sativum L.) EOs on N. tenuis in terms of acute toxicity and sublethal effects (Papadimitriou et al. 2019; Soares et al. 2019; Campolo et al 2020b; Ricupero et al. 2022). Most of these studies evaluated EO side effect by testing the pest recommended label rate or the lethal concentrations (LCs) estimated on the target pest. However, there is a lack of data concerning the dose–response relationship of EOs on N. tenuis and their effects at low concentrations (i.e. that cause low mortality) on the fertility and orientation capacity of this predator.
The aim of this study was to assess in the laboratory the baseline toxicity caused by different concentrations of four commercial EOs on the predator N. tenuis. We also investigated the behavioral and physiological response of the predator under the effect of these compounds in low concentrations.
Materials and methods
Biological materials
The N. tenuis individuals used in the bioassays were obtained in the laboratory rearing according to the methodology described by Passos et al. (2022). Adults of N. tenuis (~ 150 individuals) were kept in entomological cages (32 × 40 × 70 cm) covered by fine net mesh. Potted seedlings (~ 30 cm high) of Sesamum indicum L. (variety T-85 Humera) were added to the cages as water and oviposition sources, and the commercial mixture of the alternative prey Ephestia kuehniella Zeller (Lepidoptera: Pyralidae) eggs and Artemia spp. cysts (i.e., Entofood®, Koppert, the Netherlands) was offered ad libitum to the predators (spread over the leaves) as an additional food source (Biondi et al. 2016). Individuals were maintained in entomological cages for three days for mating and oviposition, and subsequently they were collected with a mechanical aspirator and transferred into new cages as previously described. The sesame seedlings containing N. tenuis eggs were kept in the cages for egg hatching and the development of newly hatched nymphs until adulthood. Insect rearing and experiments were carried out under laboratory controlled conditions (25 ± 1 °C, 55 ± 5% RH, and 14L:10D photoperiod) at the Department of Agriculture, Food and Environment of the University of Catania (Italy).
EO nanoemulsions preparation and characterization
Anise (Pimpinella anisum L.-Apiaceae), fennel (Foeniculum vulgare Mill.-Apiaceae), lavender (Lavandula angustifolia Miler.-Lamiaceae) and garlic (A. sativum) commercial food grade EOs (Esperis s.p.a. Milano, Italy) were used to develop insecticide nanoemulsions. For complete analytical procedures and chemical characterization (GC/FID and GC/MS analyses) of anise, fennel, lavender EOs see Campolo et al. (2020a), and for garlic EO see Ricupero et al. (2022).
All the developed EO-based nanoemulsions were obtained following the procedures proposed by Giunti et al. (2019). In details, 15% of each EO and 5% Tween 80 (w/w) were mixed for 30 min (8000 RPM) using a magnetic stirrer; then distilled water (80%) was dropwise (1 mL × min−1) added and then stirred for 60 min. To reduce the particle size and improve the stability, the resulted raw emulsions were then sonicated for 5 min using an UP200ST ultrasonic immersion homogenizer (Hielsher©, Teltow, Germany) at 100 W power (frequency: 26 kHz) (Campolo et al. 2020a). The physical characterization of the developed formulations was carried out by a dynamic light scattering (DLS) apparatus (Zetasizer Nano, Malvern). The quality and the stability patterns over time of the developed EO-based nanoformulations were evaluated through the measurement of the following parameters: droplet dimension, expressed in terms of Z-average size (nm), polydispersity index (PDI) and zeta (ζ) potential (droplet surface charge expressed as mV).
Baseline toxicity of EOs toward Nesidiocoris tenuis females
The concentration-mortality bioassay aimed at assessing the topical contact toxicity of anise, fennel, lavender and garlic EOs on N. tenuis adult females. Nesidiocoris tenuis females were topically exposed from five to six serial dilutions of each EO completely dispersed in a solution of distilled water. The EO dilutions were chosen according to preliminary observations aimed at identifying the minimum concentration necessary to cause 100% mortality of N. tenuis females and the maximum concentration that cause no mortality similarly to the untreated control. Additionally, a stock solution containing 15% of EO (i.e., concentrated nanoemulsion) was used as the highest concentration, and an untreated control with only distilled water was included for all the treatments as the “zero concentration”.
For the bioassay, N. tenuis females (2-day old) from the laboratory rearing were isolated in groups of five individuals in conical ventilated plastic tubes (Falcon®—50 mL) and maintained at low temperature (~ 8 °C) inside a thermic box containing ice packs for 3 h to reduce their mobility. Thereafter, each group of N. tenuis females was placed in a plastic cup (100 mL) and immediately sprayed with 2 mL of each concentration of EO solutions through a hand-sprayer (50 mL). The inner part of plastic cups was covered in absorbent paper to avoid the formation of solution droplets and the potential drowning of sprayed insects. The absorbent paper was replaced among replicates for each EO-concentration. After spraying, each group of N. tenuis females was transferred in an acrylic ventilated arena (5.5 cm diameter, 3 cm ht) containing Entofood® (scattered on the arena) and a zucchini leaf disc (3 cm in diameter) as food and water sources. The number of dead females was recorded after 48 h. Each arena containing five N. tenuis females was considered a replicate and the experiment was replicated eight times per each EO tested concentration and the untreated control.
Effects of EOs on Nesidiocoris tenuis fertility
This bioassay aimed at evaluating whether previously determined decreasing EO concentrations can affect the progeny production of N. tenuis females. Predators were exposed to LC1, LC10 and LC30 of anise, fennel, lavender and garlic EOs in order to emulate a potential field scenario in which different insecticide concentration may occur in field after environmental degradation, including a lethal range from negligible mortality (LC1) to moderate mortality (LC30).
Nesidiocoris tenuis adults (2 days old) were sprayed with LC1, LC10 and LC30 of anise, fennel, lavender and garlic EOs in the same process previously described in “Baseline toxicity of EOs toward Nesidiocoris tenuis females”. Thereafter, each N. tenuis male and female was placed into a ventilated arena (400 mL) containing 1 g of E. kuehniella eggs as food supply and a green bean pod (Phaseolus vulgaris L., cv. “Garrafal enana”) as oviposition substrate (Passos et al. 2022). Nesidiocoris tenuis couples were kept into experimental arenas to mate successfully and allow females to oviposit into green bean pods. After 3 days, N. tenuis adults were removed from the arenas. Green bean pods bearing N. tenuis eggs were thus maintained at the aforementioned described controlled laboratory conditions. Approximately ten days after, newly emerged N. tenuis nymphs were daily counted (= fertility) and removed with a soft paintbrush under the stereomicroscope. The evaluation was conducted for 20 days until no nymph emerged. For each EO-concentration combination and the control, the fertility of 25 N. tenuis females (each female being a replicate) was evaluated.
Effects of EOs on Nesidiocoris tenuis orientation
The aim of this bioassay was to assess the potential effects of EOs at low-lethal concentrations on the orientation capacity of N. tenuis females. For this, adult females (~ 2 days old) were topically exposed to LC1, LC10 and LC30 of anise, fennel, lavender and garlic EOs following the same methodology described in “Baseline toxicity of EOs toward N tenuis females”. Briefly, N. tenuis females collected from the rearing were starved for 24 h in transparent vials with a moistened cotton wad as water source. Thus, after being sprayed topically, each N. tenuis female was individually transferred into a two-way olfactometer (main arm and lateral arms 15 cm long and 4 cm internal diameter) and observed. The odor sources were clean air and a sesame plant (~ 20 cm height), since prior studies reported high attractiveness of this plant species to N. tenuis (Biondi et al. 2016). Sesame plant was placed inside one of the cylindrical glass jars (5 L) connected to the lateral arms of the olfactometer. An air pump (Airfizz®, Ferplast, Italy) produced a unidirectional flow (150 mL× min−1) that passed through a water filter before entering the olfactometer system, conducting the air through the olfactometer lateral arms and reaching the main arm. The olfactometer was placed vertically on the bench surface and illuminated by 22 W cool-white fluorescent lamps positioned 80 cm above. The observations were conducted between 9:00 a.m. and 6:00 p.m. in a dark room at the aforementioned laboratory conditions. The olfactometer was inverted for eliminating environmental interference to insect response every two replicates. Both the olfactometer and glass jars were cleaned with pure grade acetone between the tested EOs and their different concentrations.
Each N. tenuis female was observed for 5 min and the time spent by the predator for choosing between the odor sources was recorded. The choice was considered valid when each N. tenuis female crossed 2/3 of the lateral arm. Per each EO-concentration combination 30 valid replicates, i.e., females that made a choice within the 5 min of observations, were considered.
Statistical analyses
The dependent variables (i.e., mortality, fertility and olfactory response data) were checked for normality of variance and homoscedasticity through Shapiro’s test and Wilk’s test, respectively, and dataset was log-transformed whenever needed. The baseline toxicity of anise, fennel, lavender and garlic EOs on N. tenuis by topical contact exposure was determined by a log-probit regression model. The concentration-mortality relationships were considered valid when no significant deviation occurred between the observed and the expected values at p < 0.05 level. The preference of N. tenuis females that made a choice between sesame plant and clean air was analyzed through the chi-squared goodness-of-fit to determine whether the orientation behavior toward each odor source diverged from an equal (50:50) distribution. The effect of the factors “EO”, “Concentration” and their interaction “EO” × “Concentration” on mortality, fertility and time spent data was analyzed through a Generalized Linear Model (GLM). Means were separated by a post-hoc Tukey HSD test. Probit analyses were carried out in IBM® SPSS® Statistics for Macintosh, Version 23.0.0.0 (IBM Corp. Released 2015. Armonk, NY: IBM CorpSPSS v. 21.0). Statistical analyses for fertility and olfactory response data were performed in “R” 3.6.0 (R Core Team 2019), using the packages “car” and “MASS” for model fitting and the package “multcomp” to separate means.
Results
Baseline toxicity of EOs to Nesidiocoris tenuis females
Probit models fit the observed data for all treatments (p > 0.05), validating the concentration–response curve and therefore estimated low-lethal concentrations for all EOs (Table 1). During the evaluation period, no death was recorded in insects treated only with distilled water (“zero concentration”). The lowest low-lethal concentrations were estimated for garlic EO which hence presented the highest toxicity toward N. tenuis females. The highest LC1 and LC10 were estimated for anise EO, while the highest LC30 was recorded for fennel EO.
Effects of EOs on Nesidiocoris tenuis fertility
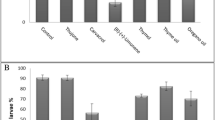
There was a significative interaction between “EO” × “Concentration” (χ2 = 22.25, df = 8, p < 0.001), indicating a higher reduction in N. tenuis fertility when EO concentration increased. For all EOs, the reduction in fertility was higher at LC30 (progeny/female of 1.33, 1.25, 1.20 and 1.36 for anise, garlic, fennel and lavender EOs, respectively; while the progeny for untreated control was 7.89) in comparison with LC1 and LC10 (progeny/female of 3.42, 2.33, 2.25 and 2.78 at LC1 and 3.00, 3.73, 3.06 and 2.93 at LC10 for anise, garlic, fennel and lavender EOs, respectively). Within the three concentrations, treatments with EOs significantly reduced the fertility of N. tenuis females in comparison with the untreated control (progeny/female of 7.35, 8.17 and 7.89 for the tests within LC1, LC10 and LC30) (Fig. 1).
Mean number (± SE) of nymphs generated by Nesidiocoris tenuis females during three days after being topically exposed to anise, fennel, garlic and lavender essential oils at three low-lethal concentrations (LC1, LC10 and LC30). Different capital letters indicate significant differences among treatments in a concentration, while different lower-case letters indicate significant differences in the concentrations for a treatment (GLM—Poisson distribution, Tukey HSD test, p ≤ 0.05)
Effects of EOs on Nesidiocoris tenuis orientation
In the untreated control N. tenuis females showed significant attraction toward sesame plants rather than clean air. Conversely, all three low-lethal estimated concentrations of lavender, fennel and garlic EOs affected the attraction of N. tenuis females toward sesame plants, resulting in no difference in the choices between sesame and clean air. The orientation capacity of N. tenuis females sprayed with anise EO was affected only at its LC30 (Fig. 2).
Olfactory response of Nesidiocoris tenuis females topically exposed to anise, fennel, garlic and lavender essential oils at three low-lethal concentrations, (LC1, LC10 and LC30 toward Sesamum indicum emitted volatiles and clean air. “n.c.” = no choice, indicate the number of non-responder females. “n” = N. tenuis females making a choice within the 5 min of observations. Asterisks indicate significant differences in the attraction toward S. indicum plant and clean air (likelihood chi-squared test p ≤ 0.05)
The time spent by N. tenuis females for choosing between the odor sources was also significantly affected by the EOs in LC1 (χ2 = 16.29, df = 4, p < 0.001) and LC10 (χ2 = 12.23, df = 4, p = 0.002). Nesidiocoris tenuis females treated with garlic EO significantly spent more time for choosing in comparison with untreated insects. In LC30, all EOs increased the time spent by insects to make a choice in comparison with untreated insects, except the anise EO (χ2 = 28.92, df = 4, p < 0.001). Regarding each EO, time spent by N. tenuis females to make a choice was not affected by the concentration for anise (χ2 = 2.25, df = 2, p = 0.320), garlic (χ2 = 1.24, df = 2, p = 0.540) and fennel (χ2 = 4.64, df = 2, p = 0.100); however, for lavender an increase was observed at LC30 in comparison with LC1 (χ2 = 6.04, df = 2, p = 0.040) (Fig. 3). Nevertheless, no interaction among EOs and their concentrations was highlighted in N. tenuis choice time between the two odor sources (χ2 = 9.39, df = 8, p = 0.310).
Discussion
The interest of EOs as pest control tools has been increased in the past decades. These compounds are considered eco-friendly, with low impact on the environment and low toxicity to mammalians. EOs derived from plants of different species are have been studied in combination with different formulation technologies to obtain more stable and efficient plant protection products, in order to be incorporated in pest management programs (Campolo et al. 2017; 2020a). In our experiment, we found various toxicity levels caused by EOs toward N. tenuis behavior (orientation) and physiology (reproduction).
Among the tested EOs, garlic EO was the most toxic compound with the lowest lethal concentrations estimated. It also negatively affected the progeny produced by N. tenuis females especially that the concentration of LC30. Also, the three tested LCs of garlic EO impaired the orientation capacity of N. tenuis since the predator spent significantly more time and it was not able to distinguish between attractive and non-attractive odor source. Although, the insecticidal properties of garlic EO have been widely recognized for numerous insect pests (Regnault-Roger 1997; Ricupero et al. 2022) and several commercial formulations are currently marketed in different world countries (Anwar et al. 2014), to the best of our knowledge, we reported for the very first the side effect of garlic EO on the behavior of a zoophytophagous mirid predator.
The high toxicity recorded for garlic EO can be attributed to its composition consisting in more than 90% of organosulfides. Several studies report the acute toxicity and sublethal effects of garlic EO and its components on different development stages of arthropod pests (Ho et al. 1996; Meriga et al. 2012; Yang et al. 2012; Plata-Rueda et al. 2017). The same nanoemulsioned garlic EO of the current study at the concentrations of 0.12 and 3% was effective in controlling T. absoluta but reduced the fertility in N. tenuis, although low mortality of the predator occurred (Ricupero et al. 2022). The difference in the EO toxicity might be related to different exposure route, i.e., topical contact in the present study, while previously dry residue was the exposure route. Conversely, although topical application of sublethal doses of garlic extract disrupted some reproductive life table parameters of the predatory bug Podisus maculiventris (Say) (Hemiptera: Pentatomidae), this compound was considered a safe botanical when the predator plays a key role in pest control (Mamduh et al. 2017). In this case, different concentrations and/or diverse compounds present in different types of garlic extracts might be related to the changing toxicity.
Anice EO showed the highest LCs (lower toxicity) among the tested EOs but decreased the fertility and the orientation capacity of N. tenuis, with the exception of LC10 and LC1 for the olfactory response of the predator. Acute toxicity by anise EO on N. tenuis might be due to the high proportion of phenylpropanoids with anethole and estragole constituting approximately the 80% in oil composition. Toxic effects of anice EO in the literature include increase in mortality of Tribolium castaneum (Herbst) (Coleoptera: Tenebrionidae), reduction in progeny at increasing concentrations, irritations and damages to different body parts, including cells of the midgut, which was due probably to its lipophilicity (Hashem et al. 2018). Similar acute toxicity and repellency against Tribolium confusum were recorded for nanoemulsioned anice EO delivered by cold aerosol and gel formulation in laboratory trials (Palermo et al. 2021). Conversely, testing the anise EO in the laboratory, Benelli et al. (2018) detected scarce and no toxicity on larvae and adults of the aphid predator Harmonia axyridis (Pallas) (Coleoptera: Coccinellidae), respectively.
Among the EOs evaluated in this experiment, topical application of lavender EO showed an intermediate toxicity toward N. tenuis, resulting in progeny reduction and adverse effect on its orientation at the olfactory bioassay. The toxicity of lavender EO has been demonstrated in the laboratory by topical application on several agricultural and veterinary pests including Hemiptera (Faraone et al. 2015; Grul'ová et al. 2017) and Diptera (Shalaby et al. 2016). Amer et al. (2002) observed that French lavender EO decreased the oviposition rate and food consumption of Amblyseius swirskii Athias-Henriot (Acari: Phytoseiidae), albeit the EO was considered less toxic toward the predacious mite. The toxicity of lavender EO has been attributed to linalool (Erland et al. 2015), its major component with recognized acetylcholinesterase inhibition activity; linalool constituted over the 40% of the tested lavender EO.
Lethal toxicity on N. tenuis caused by fennel EO in its lower LCs was slightly lower in comparison with garlic EO but higher if compared to anise and lavender EOs. In our laboratory trials, fennel EO also affected N. tenuis progeny production as also recorded for the other differently concentrated EOs we tested. Besides, N. tenuis females were not able to discriminate between the attractive and non-attractive odor sources when treated topically with different LCs of fennel EO and they spent more time for choosing under the influence of LC30. The insecticidal properties of fennel EO have recognized for its insecticide effects against many insects, such as Spodoptera littoralis (Boisduval) (Lepidoptera: Noctuidae), larvae of Culex quinquefasciatus Say (Diptera: Culicidae), adults of Musca domestica Linnaeus (Diptera: Muscidae) (Pavela et al. 2016), including Acyrthosiphon pisum (Harris) (Hemiptera: Aphididae) and Myzus persicae (Sulzer) (Hemiptera: Aphididae) (Digilio et al. 2008). Pavela (2018) found that fennel EO showed toxicity on M. persicae in the range of 0.3–3.7 mL × L−1 but, these concentrations had no mortality toward the predator H. axyridis. The most present compound in fennel EO is cis-anethole, a phenylpropanoid that can cause insecticidal effect. Phenylpropanoids can neutralize insect defense mechanisms, i.e., P450, glutathione-S-transferases and esterases (Jankowska et al. 2018). Fennel EO also has a considerable proportion of monoterpenes (approximately 40%), mainly limonene and fenchone. Therefore, we have reasons to believe that the high proportion of phenylpropanoids and monoterpenes was associated with the deleterious effects observed to N. tenuis in the present experiment.
Mean (± SE) time spent (s) by Nesidiocoris tenuis females for choosing between volatiles emitted by a Sesamum indicum plant or clean air after topical contact exposure to anise, fennel, garlic and lavender essential oils at three low-lethal concentrations (LC1, LC10 and LC30). Different capital letters indicate differences among treatments in a concentration, while different lower-case letters indicate differences in the concentrations for a treatment (GLM—Negative Binomial distribution, Tukey HSD test, p ≤ 0.05)
Most EOs can potentially cause behavioral and physiological effects on insects at a given dose, however, behavioral effects are more likely to be observed at concentrations below those necessary to produce acute toxicity and secondary effects on physiology (Isman and Tak 2017). In the present bioassays, the EOs probably penetrated N. tenuis tegument due to topical treatment, causing physiological and behavioral effects at low concentrations on fertility and orientation.
Healthy insects can detect fragrant and chemosensory-active compounds such as plant volatile compounds through odorant binding proteins and chemosensory proteins, located on the periphery of sensory receptors, with the function to capture and transport molecular stimuli (Picimbon 2005). In our experiment, we were able to observe untreated N. tenuis females highly attracted to sesame plants, as previously reported (Biondi et al. 2016; Passos et al. 2022). After treatment with the EOs, even at very low concentrations, the orientation of N. tenuis toward a host plant was compromised. The neurotoxic effects of EOs might have affected the capacity of the predator to guide themselves toward the stimuli. Moreover, the direct treatment with the oils might have caused confusion to the insect, because many compounds can interfere with proteins responsible for perception.
The persistence of EOs (i.e., the time that the product remains biologically active against pests after application) is often considered low, although field trials showed that one single spray can provide up to three weeks of protection, presumably for the repellent effect of residual concentrations (Isman et al. 2011). Low persistence of EOs in the environment is due to their high volatility (Hu and Coats 2008; Rathore 2017). Nanotechnology can improve their stability patterns, susceptibility to oxidation and prevent the phytotoxicity (Rathore 2017; Isman 2020). By contrast, for zoophytopaghous mirids which may also survive on host plants such as N. tenuis, the increased persistence provided by the nanoformulation can be harmful, since the deleterious effects of EOs might be prolonged. The contact with lower concentrations is likely to happen in field conditions even when higher doses of insecticides are used, due to natural degradation of the compounds (Eijaza et al. 2015). This can negatively affect their survival, development and their efficiency as biological control agents (Desneux et al. 2007; Soares et al. 2019). As a consequence, careful evaluation should be taken before using nanoemulsioned EOs when the predation exerted by N. tenuis is desirable. However, in situation when N. tenuis populations need to be reduced, namely when prey is lacking and injury on tomato plants may occur (Castañé et al. 2011), the EOs can be used as a management strategy to mitigate plant damage by N. tenuis.
In conclusion, we observed that N. tenuis was susceptible to anise, fennel, garlic and lavender EOs even in low concentrations since these compounds negatively affected its fertility and orientation behavior, with garlic and lavender EOs being the most harmful compounds. Therefore, the use of these EOs should be carefully evaluated when the presence of N. tenuis is suitable because they might impair the predator’s capacity to efficiently exert biological control activity.
Future studies on the insect olfactory perception under the influence of EOs through electroantennography or transcriptome analyses are warranted to unveil unknown mechanisms that cause detrimental effects on insect nervous system (Campolo et al. 2020b). The assessment of reproductive parameters of the mirid predator under the influence of EOs in combination with environmental stressors is another point that should be considered (Ricupero et al. 2020). Finally, spraying of these EOs under field conditions should be carried in order to assess how the biological control provided by N. tenuis is affected by these compounds. Overall, EOs are likely to be increasingly used in the future, accelerating changes in the way pest control is provided. For this reason, researchers and policy makers have thus an exciting and challenging opportunity to use them to improve the IPM systems worldwide.
Author contributions
LCP: contributed to conceptualization, methodology, execution of experiments, data collect, statistical analysis, investigation, writing; MR: contributed to conceptualization, methodology, execution of experiments, data collect, statistical analysis, investigation, writing; AG: contributed to conceptualization, methodology, execution of experiments, data collect, statistical analysis, investigation, writing; MAS: contributed to statistical analysis, writing—review and editing; ND: contributed to conceptualization, writing—review and editing; OC: contributed to conceptualization, writing—review and editing; GAC: contributed to conceptualization, writing—review and editing; AB: contributed to conceptualization, methodology, supervision, writing—review and editing; LZ: contributed to conceptualization, writing—review and editing, project administration.
Data availability
Not applicable.
Change history
11 September 2022
The original online version of this article was revised : the article category has been updated.
References
Amer SAA, Momen FM (2002) Effect of some essential oils on the predacious mite Amblyseius swirskii AH (Acari: Phytoseiidae). Acta Phytopathol Entomol Hung 37:281–286. https://doi.org/10.1556/APhyt.37.2002.1-3.27
Anwar A, Groom M, Arbach M, Hamilton CJ (2014) How to turn the chemistry of garlic into a ‘botanical’pesticide. Recent advances in redox active plant and microbial products. Springer, Dordrecht, pp 323–341. https://doi.org/10.1007/978-94-017-8953-0_12
Benelli G, Pavela R, Petrelli R, Cappellacci L, Canale A, Senthil-Nathan S, Maggi F (2018) Not just popular spices! essential oils from Cuminum cyminum and Pimpinella anisum are toxic to insect pests and vectors without affecting non-target invertebrates. Ind Crops Prod 124:236–243. https://doi.org/10.1016/j.indcrop.2018.07.048
Biondi A, Guedes RNC, Wan FH, Desneux N (2018) Ecology, worldwide spread, and management of the invasive South American tomato pinworm, Tuta absoluta: past, present, and future. Annu Rev Entomol 63:239–258. https://doi.org/10.1146/annurev-ento-031616-034933
Biondi A, Zappalà L, Di Mauro A et al (2016) Can alternative host plant and prey affect phytophagy and biological control by the zoophytophagous mirid Nesidiocoris tenuis? Biocontrol 61:79–90. https://doi.org/10.1007/s10526-015-9700-5
Calvo FJ, Lorente MJ, Stansly PA et al (2012) Preplant release of Nesidiocoris tenuis and supplementary tactics for control of Tuta absoluta and Bemisa tabaci in greenhouse tomato. Entomol Exp Appl 143:111–119. https://doi.org/10.1111/j.1570-7458.2012.01238.x
Campolo O, Cherif A, Ricupero M et al (2017) Citrus peel essential oil nanoformulations to control the tomato borer, Tuta absoluta: chemical properties and biological activity. Sci Rep 7:13036. https://doi.org/10.1038/s41598-017-13413-0
Campolo O, Giunti G, Laigle M, Michel T, Palmeri V (2020a) Essential oil-based nano-emulsions: Effect of different surfactants, sonication and plant species on physicochemical characteristics. Ind Crop Prod 157:112935. https://doi.org/10.1016/j.indcrop.2020.112935
Campolo O, Puglisi I, Barbagallo RN et al (2020b) Side effects of two citrus essential oil formulations on a generalist insect predator, plant and soil enzymatic activities. Chemosphere 257:127252. https://doi.org/10.1016/j.chemosphere.2020.127252
Castañé C, Arnó J, Gabarra R et al (2011) Plant damage to vegetable crops by zoophytophagous mirid predators. Biol Control 59:22–29. https://doi.org/10.1016/j.biocontrol.2011.03.007
Cherif A, Mansour R, Sun C, Grissa-Lebdi K (2022) Lethal effects of nano and commercial formulations of abamectin on Tuta absoluta (Meyrick) and its mirid predators Macrolophus pygmaeus and Nesidiocoris tenuis. Intern J Trop Insect Sci. https://doi.org/10.1007/s42690-022-00739-0
de Oliveira JL, Campos EVR, Bakshi M, Abhilash PC, Fraceto LF (2014) Application of nanotechnology for the encapsulation of botanical insecticides for sustainable agriculture: prospects and promises. Biotech Adv 32:1550–1561. https://doi.org/10.1016/j.biotechadv.2014.10.010
Desneux N, Decourtye A, Delpuech JM (2007) The sublethal effects of insecticides on beneficial arthropods. Annu Rev Entomol 52:81–106. https://doi.org/10.1146/annurev.ento.52.110405.091440
Desneux N, Han P, Mansour R et al (2021) Integrated pest management of Tuta absoluta: practical implementations across different world regions. J Pest Sci 95:17–39. https://doi.org/10.1007/s10340-021-01442-8
Digilio MC, Mancini E, Voto E, De Feo V (2008) Insecticide activity of Mediterranean essential oils. J Plant Interact 3:17–23. https://doi.org/10.1080/17429140701843741
Dunan L, Malanga T, Bearez P, Benhamou S et al (2021) Biopesticide evaluation from lab to greenhouse scale of essential oils used against Macrosiphum euphorbiae. Agriculture 11(9):867. https://doi.org/10.3390/agriculture11090867
Eijaza S, Khan MF, Mahmood K, Anwar M, Alamgir A, Khatri I (2015) Studies on degradation and efficacy of synthetic pesticides on okra crop. Acad J Entomol 8:12–18
Erland LAE, Rheault MR, Mahmoud SS (2015) Insecticidal and oviposition deterrent effects of essential oils and their constituents against the invasive pest Drosophila suzukii (Matsumura) (Diptera: Drosophilidae). Crop Prot 78:20–26. https://doi.org/10.1016/j.cropro.2015.08.013
Faraone N, Hillier NK, Cutler GC (2015) Plant essential oils synergize and antagonize toxicity of different conventional insecticides against Myzus persicae (Hemiptera: Aphididae). PLoS ONE 10:e0127774. https://doi.org/10.1371/journal.pone.0127774
Giunti G, Palermo D, Laudani F, Algeri GM, Campolo O, Palmeri V (2019) Repellence and acute toxicity of a nano-emulsion of sweet orange essential oil toward two major stored grain insect pests. Ind Crop Prod 142:111869. https://doi.org/10.1016/j.indcrop.2019.111869
Grul’ová D, Mudrončeková S, Zheljazkov VD, Šalamon I, Rondon SI (2017) Effect of plant essential oils against Rophalosiphum padi on wheat and barley. Nat Prod Commun 12:1517–1520. https://doi.org/10.1177/1934578X1701200933
Hare JD (2011) Ecological role of volatiles produced by plants in response to damage by herbivorous insects. Annu Rev Entomol 56:161–180. https://doi.org/10.1146/annurev-ento-120709-144753
Hashem AS, Awadalla SS, Zayed GM, Maggi F, Benelli G (2018) Pimpinella anisum essential oil nanoemulsions against Tribolium castaneum - insecticidal activity and mode of action. Environ Sci Pollut Res 25:18802–18812. https://doi.org/10.1007/s11356-018-2068-1
Ho SH, Koh L, Ma Y, Huang Y, Sim KY (1996) The oil of garlic, Allium sativum L. (Amaryllidaceae), as a potential grain protectant against Tribolium castaneum (Herbst) and Sitophilus zeamais Motsch. Postharvest Biol Tech 9:41–48. https://doi.org/10.1016/0925-5214(96)00018-X
Hu D, Coats J (2008) Evaluation of the environmental fate of thymol and phenethyl propionate in the laboratory. Pest Manag Sci 64:775–779. https://doi.org/10.1002/ps.1555
Ingegno BL, Messelink GJ, Leman A, Sacco D, Tavella L (2021) Development and thermal activity thresholds of European mirid predatory bugs. Biol Control 152:104423. https://doi.org/10.1016/j.biocontrol.2020.104423
Isman MB, Miresmailli S, Machial C (2011) Commercial opportunities for pesticides based on plant essential oils in agriculture, industry and consumer products. Phytochem Rev 10:197–204. https://doi.org/10.1007/s11101-010-9170-4
Isman MB, Tak JH (2017) Commercialization of insecticides based on plant essential oils: Past, present, and future. In: Nollet LM, Rathore HS (eds) Green pesticides handbook: Essential oils for pest control. CRC Press, Boca Raton, FL, pp 27–42. https://doi.org/10.1201/9781315153131-2
Isman MB (2020) Botanical insecticides in the twenty-first century-fulfilling their promise? Annu Rev Entomol 65:233–249. https://doi.org/10.1146/annurev-ento-011019-025010
Jankowska M, Rogalska J, Wyszkowska J, Stankiewicz M (2018) Molecular targets for components of essential oils in the insect nervous system - a review. Molecules 23:34. https://doi.org/10.3390/molecules23010034
Kah M, Beulke S, Tiede K, Hofmann T (2013) Nanopesticides: state of knowledge, environmental fate, and exposure modeling. Crit Rev Environ Sci Technol 43:1823–1867. https://doi.org/10.1080/10643389.2012.671750
Kim S, Yoon J, Tak JH (2021) Synergistic mechanism of insecticidal activity in basil and mandarin essential oils against the tobacco cutworm. J Pest Sci 94(4):1119–1131. https://doi.org/10.1007/s10340-021-01345-8
Krzyżowski M, Baran B, Łozowski B, Francikowski J (2020) The role of dilution mediums in studies of fumigant insecticidal activity of essential oils. J Pest Sci 93:1119–1124. https://doi.org/10.1007/s10340-020-01241-7
Madbouni MAZ, Samih MA, Namvar P, Biondi A (2017) Temperature-dependent functional response of Nesidiocoris tenuis (Hemiptera: Miridae) to different densities of pupae of cotton whitefly, Bemisia tabaci (Hemiptera: Aleyrodidae). Europ J Entomol 114:325. https://doi.org/10.14411/eje.2017.040
Mamduh Z, Hosseininaveh V, Allahyari H, Talebi-Jahromi K (2017) Side effects of garlic extract on the life history parameters of the predatory bug, Podisus maculiventris (Say)(Hemiptera: Pentatomidae). Crop Prot 100:65–72. https://doi.org/10.1016/j.cropro.2017.05.029
Mansour R, Biondi A (2021) Releasing natural enemies and applying microbial and botanical pesticides for managing Tuta absoluta in the MENA region. Phytoparasitica 49:179–194. https://doi.org/10.1007/s12600-020-00849-w
Maroofpour N, Mousavi M, Hejazi MJ et al (2021) Comparative selectivity of nano and commercial formulations of pirimicarb on a target pest, Brevicoryne brassicae, and its predator Chrysoperla carnea. Ecotoxicology 30(2):361–372. https://doi.org/10.1007/s10646-021-02349-x
Meriga B, Mopuri R, MuraliKrishna T (2012) Insecticidal, antimicrobial and antioxidant activities of bulb extracts of Allium sativum. Asian Pac J Trop Med 5:391–395. https://doi.org/10.1016/S1995-7645(12)60065-0
Miresmailli S, Isman MB (2014) Botanical insecticides inspired by plant–herbivore chemical interactions. Trends Plant Sci 19:29–35. https://doi.org/10.1016/j.tplants.2013.10.002
Moretti MDL, Sanna-Passino G, Demontis S, Bazzoni E (2002) Essential oil formulations useful as a new tool for insect pest control. AAPS Pharm SciTech 3:64–74. https://doi.org/10.1208/pt030213
Naselli M, Biondi A, Tropea Garzia G et al (2017) Insights into food webs associated with the South American tomato pinworm. Pest Manag Sci 73(7):1352–1357. https://doi.org/10.1002/ps.4562
Palermo D, Giunti G, Laudani F, Palmeri V, Campolo O (2021) Essential oil-based nano-biopesticides: formulation and bioactivity against the confused flour beetle Tribolium confusum. Sustainability 13:9746. https://doi.org/10.3390/su13179746
Papadimitriou DM, Petrakis EA, Arvaniti KA et al (2019) Comparative bioactivity of essential oils from two Mentha pulegium (Lamiaceae) chemotypes against Aphis gossypii, Aphis spiraecola, Tetranychus urticae and the generalist predator Nesidiocoris tenuis. Phytoparasitica 47:683–692. https://doi.org/10.1007/s12600-019-00770-x
Passos LC, Ricupero M, Gugliuzzo A et al (2022) Does the dose make the poison? Neurotoxic insecticides impair predator orientation and reproduction even at low concentrations. Pest Manag Sci 78:1698–1706. https://doi.org/10.1002/ps.6789
Pavela R (2018) Essential oils from Foeniculum vulgare Miller as a safe environmental insecticide against the aphid Myzus persicae Sulzer. Environ Sci Pollut Res 25:10904–10910. https://doi.org/10.1007/s11356-018-1398-3
Pavela R, Žabka M, Bednář J, Tříska J, Vrchotová N (2016) New knowledge for yield, composition and insecticidal activity of essential oils obtained from the aerial parts or seeds of fennel (Foeniculum vulgare Mill.). Ind Crop Prod 83:275–282. https://doi.org/10.1016/j.indcrop.2015.11.090
Pavela R, Morshedloo MR, Mumivand H, Khorsand GJ et al (2020) Phenolic monoterpene-rich essential oils from Apiaceae and Lamiaceae species: insecticidal activity and safety evaluation on non-target earthworms. Entomol Gen 40:421–435. https://doi.org/10.1127/entomologia/2020/1131
Pérez-Hedo M, Riahi C, Urbaneja A (2021a) Use of zoophytophagous mirid bugs in horticultural crops: current challenges and future perspectives. Pest Manag Sci 77:33–42. https://doi.org/10.1002/ps.6043
Pérez-Hedo M, Alonso-Valiente M, Vacas S, Gallego C et al (2021b) Eliciting tomato plant defenses by exposure to herbivore induced plant volatiles. Entomol Gen 41(3):209–218. https://doi.org/10.1127/entomologia/2021/1196
Picimbon JF (2005) Synthesis of odorant reception-suppressing agents, odorants-binding proteins (OBPs) and chemosensory proteins (CSPs): molecular targets for pest management. In: Regnault-Roger C, Philogène BJR, Vincent C (eds) Biopesticides of plant origin. Lavoisier, Paris, pp 383–416
Plata-Rueda A, Martínez LC, Dos Santos MH et al (2017) Insecticidal activity of garlic essential oil and their constituents against the mealworm beetle, Tenebrio molitor Linnaeus (Coleoptera: Tenebrionidae). Sci Rep 7:4640. https://doi.org/10.1038/srep46406
R Development Core Team (2019) R: A language and environment for statistical computing (Version 3.6.0). R Foundation for Statistical Computing, Vienna. https://www.r-project.org/
Rathore LM (2017) Green pesticides for organic farming: occurrence and properties of essential oils for use in pest control. In: Nollet LM, Rathore HS (eds) Green pesticides handbook: essential oils for pest control. CRC Press, Boca Raton, FL, pp 3–26. https://doi.org/10.1201/9781315153131-1
Regnault-Roger C (1997) The potential of botanical essential oils for insect pest control. Integr Pest Manag Rev 2:25–34. https://doi.org/10.1023/A:1018472227889
Regnault-Roger C, Vincent C, Arnason JT (2012) Essential oils in insect control: low-risk products in a high-stakes world. Annu Rev Entomol 57:405–424. https://doi.org/10.1146/annurev-ento-120710-100554
Ricupero M, Abbes K, Haddi K et al (2020) Combined thermal and insecticidal stresses on the generalist predator Macrolophus pygmaeus. Sci Tot Environ 729:138922. https://doi.org/10.1016/j.scitotenv.2020.138922
Ricupero M, Biondi A, Cincotta F et al (2022) Bioactivity and physico-chemistry of garlic essential oil nanoemulsion in tomato. Entomol Gen. https://doi.org/10.1127/entomologia/2022/1553
Shalaby HA, El Khateeb RM, El Namaky AH et al (2016) Larvicidal activity of camphor and lavender oils against sheep blowfly, Lucilia sericata (Diptera: Calliphoridae). J Parasit Dis 40:1475–1482. https://doi.org/10.1007/s12639-015-0715-8
Siscaro G, Lo Pumo C, Tropea Garzia G et al (2019) Temperature and tomato variety influence the development and the plant damage induced by the zoophytophagous mirid bug Nesidiocoris tenuis. J Pest Sci 92:1049–1056. https://doi.org/10.1007/s10340-019-01096-7
Sciortino M, Scurria A, Lino C, Pagliaro M, D’Agostino F, Tortorici S, Ricupero M, Biondi A, Zappalà L, Ciriminna R (2021) Silica-microencapsulated orange oil for sustainable pest control. Adv Sustain Syst 5:2000280. https://doi.org/10.1002/adsu.202000280
Soares MA, Campos MR, Passos LC et al (2019) Botanical insecticide and natural enemies: a potential combination for pest management against Tuta absoluta. J Pest Sci 92:1433–1443. https://doi.org/10.1007/s10340-018-01074-5
Thomine E, Jeavons E, Rusch A, Bearez P, Desneux N (2020) Effect of crop diversity on predation activity and population dynamics of the mirid predator Nesidiocoris tenuis. J Pest Sci 93(4):1255–1265. https://doi.org/10.1007/s10340-020-01222-w
Tortorici S, Cimino C, Ricupero M et al (2022) Nanostructured lipid carriers of essential oils as potential tools for the sustainable control of insect pests. Ind Crops Prod 181:114766. https://doi.org/10.1016/j.indcrop.2022/114766
van Lenteren JC, Lanzoni A, Hemerik L et al (2021) The pest kill rate of thirteen natural enemies as aggregate evaluation criterion of their biological control potential of Tuta absoluta. Sci Rep 11:1–13. https://doi.org/10.1038/s41598-021-90034-8
Walling LL (2000) The myriad plant responses to herbivores. J Plant Growth Regul 19:195–216. https://doi.org/10.1007/s003440000026
Yang FL, Zhu F, Lei CL (2012) Insecticidal activities of garlic substances against adults of grain moth, Sitotroga cerealella (Lepidoptera: Gelechiidae). Insect Sci 19:205–212. https://doi.org/10.1111/j.1744-7917.2011.01446.x
Zappalà L, Biondi A, Alma A et al (2013) Natural enemies of the South American moth, Tuta absoluta, in Europe, North Africa and Middle East, and their potential use in pest control strategies. J Pest Sci 86(4):635–647. https://doi.org/10.1007/s10340-013-0531-9
Acknowledgements
The authors would like to thank the owner of owners of the Azienda Agricola La Zagara (Fiumefreddo, Italy) for sharing their tomato crop for insect collection, students and technicians of the Applied Entomology section of the Department Agriculture, Food and Environment of the University of Catania that contributed to the experimental execution.
Funding
Open access funding provided by Università degli Studi di Catania within the CRUI-CARE Agreement. The authors acknowledge the “Coordenação de Aperfeiçoamento de Pessoal de Nível Superior” (CAPES) for the author personal funding (PDSE—Process nº 88881.187337/2018-01), the “Fundação de Amparo à Pesquisa do Estado de Minas Gerais” (FAPEMIG) and the “Conselho Nacional de Desenvolvimento Científico e Tecnológico” (CNPq) for supporting LCP and GAC; the University of Catania (Project Emergent Pests and Pathogens and Relative Sustainable Strategies—5A722192113; PhD fellowship to AG), the EU, Programme IEV de Coopération Transfrontalière Italie‐Tunisie 2014–2020 (Project INTEMAR‐IS_2.1_073 Innovations dans la lutte intégrée contre les ravageurs et maladies récemment introduits sur cultures maraîchères, Grant Number E64I18002460007).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
AB and ND are Editor-in-Chief of Journal of Pest Science and were not involved in the journal’s review of, or decision related to, this manuscript.
Conflict of interest
The authors declare no conflict of interests. AB and ND serve as Editor-in-Chief, and OC serves as subject editor of Journal of Pest Science and were not involved in the journal’s review of, or decision related to, this manuscript.
Consent for publication
Not applicable.
Ethical approval
This article does not contain any studies with human participants or animals (other than insects) performed by any of the authors.
Additional information
Communicated by Alberto Urbaneja.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this article was revised : the article category has been updated.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Passos, L.C., Ricupero, M., Gugliuzzo, A. et al. Sublethal effects of plant essential oils toward the zoophytophagous mirid Nesidiocoris tenuis. J Pest Sci 95, 1609–1619 (2022). https://doi.org/10.1007/s10340-022-01548-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10340-022-01548-7