Abstract
The widespread use of pesticides along with the simplification of the landscape has had undesirable effects on agroecosystems, such as the loss of biodiversity and the associated ecosystem service biological control. How current production systems can be remodelled to allow for a re-establishment of biological pest control, while preserving productivity, is a major challenge. Here, we tested whether a combination of tools could augment or synergize biological control of insect pests in apple (Malus domestica), comprised of a tortricid pest complex, a geometrid pest complex and the rosy apple aphid. The tools aimed at disrupting mating behaviour of multiple pest species (multispecies mating disruption, “Disrupt”, MMD), attracting natural enemies (a blend of herbivory-induced volatiles, “Attract”, A), or providing refuge and rewards for a diverse insect community (perennial flower strip, “Reward”, R) over a 3-year period. Suction samples were consistently richer in generalist predators but not in parasitoids when multiple tools including MMD + A + R or MMD + A were employed. In addition, lepidopteran pest levels were significantly lower in these plots than in MMD or MMD + R at the end of the 3-year experiment. This was, however, not reflected in survival of artificially established aphid colonies. Our data indicates that multiple, complementary tools can greatly enhance natural enemy level, but also that long-term implementation is needed to fully realize the augmentatory or synergistic potential of complementary components and restore biological control as an ecosystem service of practical relevance.
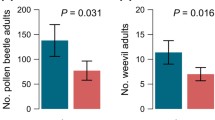
Similar content being viewed by others
Key message
-
A higher level of predators but not parasitoids was found when semiochemicals were combined with habit at manipulation
-
In the same plots, a lower damage by lepidopteran caterpillars was measured
-
This was not reflected in survival Of aphid colonies
Introduction
The intensification of agriculture over the last six decades was achieved through an exorbitant use of agrochemical inputs. Whereas this has greatly improved the gross yield, the long-term negative consequences are environmental pollution, pest resistance, loss of biodiversity along with a pronounced decrease of agroecosystem resilience (Rockstrom et al. 2017). In addition, landscape simplification accelerated a substantial decrease of local diversity in agroecosystems, which in turn, does not compensate for local high‐intensity management (Tscharntke et al. 2005). Indeed, ecosystem services and resilience is directly correlated with diversity of natural enemies and pollinators (Rusch et al. 2016). We are thus left with a major challenge to re-establish ecosystem services such as biological control in order to increase the sustainability of crop production (Tittonell 2014).
To restore ecosystem services and favour beneficials, habitat diversification has been proposed (Wilkinson and Landis 2005). This includes the introduction of resources to fulfil important needs for predators and parasitoids, such as plants providing pollen and nectar and supplementary non-pest prey or honeydew, but also a structural diversity, capable of providing shelter as well as breeding and overwintering sites. These resources are some of the key elements of conservation biological control and can be implemented at different scales (Gurr et al. 2000; Porcel et al. 2017; Wäckers et al. 2008). However, the gains are often too little to secure sufficient production. To avoid defaulting to pesticide use, additional tools need to be employed, among which semiochemicals are very promising. In, for instance, fruit and vine growing, the use of sex-pheromone mating disruption as a viable alternative has been employed for over three decades (Ioriatti and Lucchi 2016). Because sex pheromones are species-specific, additional pesticides are often required to target unaffected species that may benefit from the suppression of major pests through competitive release. The advantages provided at ecosystem level by mating disruption should thus be supported by other non-invasive methods addressing those pests not controlled by this technique.
In addition to the use of pheromones to control pests, herbivore-induced plant volatiles (HIPVs) can be used to augment ecosystem services through attracting natural enemies. Synthetic HIPVs have been released in undamaged crops to attract natural enemies from surrounding habitats to harness their effect on pests (Orre et al. 2010). However, the use of HIPVs likely needs to be complemented with resources that provide food and shelter to retain and support natural enemies, such as aforementioned non-crop vegetation. This is the basis of a novel conservation biological control approach, coined “attract and reward”, which combines two aspects of applied insect ecology: synthetic herbivore-induced plant volatiles (HIPVs) to improve immigration of beneficial taxa into crops and floral resources to maintain their populations (Simpson et al. 2011b).
Apple receives comparatively more insecticide applications per season than other crops (Reganold et al. 2001). The advantages of environmentally sustainable pest interventions can thus be particularly rewarding in this crop. Indeed, sustainable management supported beneficials quantitatively and qualitatively (Happe et al. 2019), and species diversity was 38% higher in organic as compared to conventional orchards. In addition, no trade-off between increased orchard species diversity and yield level was found (Samnegård et al. 2019). However, rebuilding resilience in our agroecosystem takes time, and needs to be supported by a diverse set of tools rather than silver bullets.
Here we hypothesized that simultaneously implementing semiochemicals and habitat diversification methods will increase the presence of natural enemies, while suppressing pests beyond a level achieved by methods singly. We deployed a combination of one habitat diversification and two chemical ecological methods in Swedish organic apple production and followed over 3 years the response of its herbivore pests (a tortricid pest complex, a geometrid pest complex, and the rosy apple aphid Dysaphis plantaginea Pass.) as well as the native natural enemies associated with these. A multispecies mating disruption served as a base method to control six tortricid species, in which flower strips (for shelter and resources of natural enemies) and HIPVs (for attracting natural enemies into the orchard) were introduced to enhance biological control of tortricids, geometrids and aphids.
Material and methods
Sites
The study was conducted over a 3-year period in five organically certified orchards in Scania (Sweden) with sizes ranging between 2.2 and 7.1 ha and a minimum separation of 2.5 km between them. Pest and natural enemy levels were assessed in plots subjected to a progressive implementation of complementary intervention techniques from year 1 to year 3, in accordance with the availability of organic orchards in the region (Fig. S1A and S2). In year 1 (2015), we compared the effect of multispecies mating disruption (MMD) with an untreated control. In the same year, flower strips were sown (hereafter named R, after “Reward”). After establishing the efficacy of MMD in 2015, in 2016, we adopted MMD as the baseline and we compared the effect on natural enemy levels and egg predation of MMD, the use of HIPVs as predator attractant (hereafter named, after “Attract”), a combo of Attract and Reward (hereafter named A + R), and MMD + R combined. This allowed us to estimate the effect of different semiochemical categories (MMD and A) on orchard arthropods. In 2017 we estimated the effect of A, R and A + R on top of MMD on the entomofauna of the orchard, on egg predation and on damage level. Four plots were established in each orchard that corresponded to a block in a randomized complete block experimental design each year (Fig. S1B).
Treatments
Multispecies mating disruption (MMD). Sex-pheromone reservoir dispensers (Isomate CLS; Shin-Etsu Ltd (Tokyo, Japan)) were applied in orchards at the density of 800 pieces per ha (See Porcel et al. 2015 for dispenser composition) for population control of Adoxophyes orana (Fischer von Röslerstamm), Archips podana (Scopoli), Archips rosana (L.), Cydia pomonella (L.), Pandemis heparana (Denis & Schiffermüller) and Spilonota ocellana (Denis & Schiffermuller). Because MMD has no reported effect on natural enemies, in experiments involving natural enemies and predation, we regarded plots with MMD as controls and did not take this treatment into consideration when combined with other treatments targeting natural enemies (e.g. MMD + R, Fig. S1B).
Reward (R). Flowers strips were sown in the drive alley between rows in 2015. Within the framework of the Eco-orchard project, a mix of 33 wild perennial species was selected based on sequential flowering during the apple growing season, long-lasting persistence, low maintenance requirements, structural diversity and accessibility of beneficial insects to nectar and pollen (Pfiffner et al. 2019). The plants selected for Nordic conditions and used in this study are provided in Table 2 from Pfiffner et al. (2019). The mix was custom prepared and provided by the company Nykilde (Slagelse, Denmark) and hand sown on a 1-m-wide soil strip at 1.8 g/m2. The mixture contained 8 grass and 25 perennial flower species in different proportions. Grass species, which accounted for 80% of the weight of the mix, provide stability against weed invasion and tractor traffic. Six and 12 weeks after sowing, the flower strips were cut at a height of 7 cm to avoid grass domination. In the following years (2016–2017), the flower strips were cut at apple pre-flowering (BBCH 57), 40% fruit development (BBCH 74) and before harvest (BBCH 85).
Attract (A). A predator attractant was purchased from Csalomon (MTA ATK, Budapest, Hungary). It consisted of a three-component blend (300 mg as total load) of methyl salicylate (MS), phenylacetaldehyde (PAA), and acetic acid (AA) in a 1:1:1 ratio on a cotton wick inside a polyethylene bag (Toth et al., 2009). These dispensers were placed on the first, central and last tree of the 5-centremost rows in the A plot. Dispensers were attached at the trunk at a height of 1.8 m. They were placed in the orchards in early May and kept for an 8-week period.
Measured variables
Pheromone trap shutdown. The flight of the six moth species was monitored through sex pheromone delta traps from May to September (See Porcel et al., 2015 for details). Because Hedya nubiferana (Haworth) damages apple inflorescence in organic orchards, traps with the corresponding sex pheromone were also set to measure the density of this non-target species. The difference in captures between the control and the disrupted plots was used as an estimate of the disruption effect.
Sentinel egg cards. To estimate biological control of tortricid eggs, sentinel egg cards were placed in the orchards. A. orana eggs were bought from Andermatt (Grossdietwil, Switzerland). Plastic strips with 1–10 eggs (mean 2.03 土 1.38) were stapled onto a 1.5 × 3.0 cm piece of cardboard. A piece of adhesive tag was used to attach the cardboard to a leaf with care not to damage the leaf and induce a localized release of HIPVs. Cards (21–30 per plot depending on the year) were stuck, one per tree, to the upper side of a leaf at 1.2 m in the middle of the 3 centre rows of each plot. After 48 h, cards were collected and the remaining eggs were counted under a stereomicroscope. The experiment consisted on two runs per year carried out in the second half of July in 2015 and 2016 and the first half of August in 2017 (Fig. S2). Cards were then placed in a rearing chamber (25 °C, 16:8 L:D period) for 60 days to estimate parasitoid emergence.
In 2016, 28 egg cards were placed at a distance of 0, 1, 2 and 4 m from the dispenser, in both directions on the four central rows of each plot to estimate the distance effect from the HIPVs. Distances were chosen according to the results obtained with a similar delivery system by Pålsson et al. (2019).
Sentinel aphid colonies. In June 2017, D. plantaginea colonies (15 per plot) were inoculated on 1-year-old shoots following the method of Porcel et al. (2018). Briefly, single females were moved from a naturally established colony to a leaf. The female was then confined within a clipcage to allow colony development in the absence of predation. After 7 days, the clipcage was removed allowing natural enemies access to the aphids. The survival of each colony was monitored weekly for 3 consecutive weeks.
Arthropods collection. Arthropods were collected from the tree canopy with a field aspirator (Bioquip Products, Rancho Dominguez, CA, USA). A sample consisted of 2 min of aspiration around the foliage on all sides of the tree and up to 2.2 m. Between 4 and 8 trees were covered in a 2-min sample depending on the tree size. Ten samples were collected in each plot twice a year to examine beneficial insects immediately after flowering and during fruit development. Samples were stored at − 18 °C for later separation of the arthropods from vegetal material and identification under stereomicroscope (Stemi SV8; Zeiss, Oberkochen, Germany). We used Salomon et al. (2000) to establish the predatory insects.
Evaluation of larval density in flower clusters. Flower clusters at the phenological development stages BBCH 59 (most flowers with petals forming a hollow ball) were sampled to evaluate emergence of pests. Data from 2015 provided the baseline before placement of any treatments. The same measurement was repeated after the 3-year experiment in 2018, to score the impact of the treatments on the overwintering population of pests (Fig. S2). This measurement was carried out at the end of the winter, prior to any use of control measure in 2018. Ten replicates were taken from the five centre rows of each plot consisting of 15 clusters each.
Establishment of flower strips. Plant species were sampled following the methodology described in Pfiffner et al. (2019). In 2017, the vegetation present in the alleyways was assessed in May, June and August using 2 × 0.6 m quadrats. Three samples were taken per treatment. The amount of plants within each quadrat were counted and determined to species level (Fig. S3).
Statistical analyses
Statistical analyses were conducted with R v3.5.2 (R Development Core Team 2016). Moth catches were analysed using generalized linear mixed models (GLMMs, package 'lme4'). The response variable was accumulated catches per orchard per species and year, while Treatment was modelled as fixed factor, and Orchard as random effect to account for the possible autocorrelation of samples from the same orchard. An initial model was tested for each species and year using a Poisson distribution, suitable for the analysis of count data. If the model was overdispersed, a negative binomial distribution was used instead to correct for overdispersion. Sentinel egg card data were submitted to binomial GLMM. Data were transformed to predation or no-predation since all cards showed either 0 or 100% predation. Baseline egg predation in 2015 was analysed alone due to no treatments affecting natural enemies established this year, with Treatment as fixed factor, and Replicate and Orchard as random effects. The 2016 and 2017 data were analysed together with Treatment and Year as fixed factors and with Replicate and Orchard as random effects. Egg predation at different distances from the centremost HIPVs dispenser was submitted to a GLMM binomial with egg predation as response variable, Treatment and Distance as fixed factors with the interaction term between them, and Orchard and Replicate as random effects. To determine how predators and parasitoids differed between treatments, GLMM models with Poisson or negative binomial error distributions for count data were used. The abundance of individual species or groups of species per plot in the different sampling months was set as the response variable, whereas Treatment and Year were set as fixed factors and Orchard as random effect. The number of leafroller and geometrid larvae on flower clusters was submitted to a GLMM with a Poisson or negative binomial distribution. Treatment, Year and their interaction were set as fixed factors and Orchard as random effect with cluster ID nested in Orchard. Geometrid and tortricid numbers were analysed separately. Tortricid groups were submitted to three different models, i.e. total tortricids (2015 and 2018), tortricids without H. nubiferana (2018) and only H. nubiferana (2018) larvae per cluster because H. nubiferana larvae were only identified in 2018. Total lepidopteran larvae per apple flower cluster were analysed with a Poisson GLMM using Treatment, and Year and their interaction as fixed factors, while Orchard was set as random effect. Cluster ID was nested within Orchard. In all GLMMs, the significance of fixed factors was tested using Wald tests with the 'car' package. Multiple comparisons between treatments were carried out following the model using a manually specified contrast matrix adjusting P-values for multiple testing with the False Discovery Rate (FDR) method with package ‘emmeans’. GLMMs were validated graphically by representing the Pearson residuals against fitted values and each of the fixed factors included in the models.
Survival of artificially established aphid colonies was analysed with a frailty model for survival data (package 'frailtypack') as described in Porcel et al. (2018). Treatment was established as fixed factor, Time as the days elapsed since aphid colony exposure to the moment of colony disappearance, and Orchard as a frailty term. The frequency of natural enemies presence in aphid colonies was analysed by means of a recurrent event survival analysis with the same structure as previously described and natural enemies presence as recurrent event.
Results
Disrupt (2015)
A. podana, H. nubiferana, P. heparana and S. ocellana were the most abundant species, whilst A. orana, A. rosana and C. pomonella were caught in lower numbers (Fig. 1). In the MMD plots, a high trap shut down was measured for all species (Fig. 1, Table S1). Egg predation on cards, regarded as a temporal baseline before the establishment of the treatments with potential positive effects on predators, ranged between 25 and 31% between the four plots, with no difference between them as expected (GLMM: df = 3, χ2 = 3.3, P = 0.352).
Average accumulated catches (± SE) of Tortricidae during 2015–2017. Bars with the same colour indicate the same experimental plot with different treatments over the years (Fig. S1B) as labelled in the x-axis. Flower strips took 1 year to establish and were thereby regarded as control the first year (2015). Bars capped with the same letter did not significantly differ within a given year (GLMM: FDR-adjusted, P < 0.05). No letters indicate no differences between treatments within a given year. MMD = Multispecies mating disruption, R = Reward, A = Attract, + indicates the combination of treatments
Attract, reward or disrupt (2016)
In 2016, some variations were recorded compared to 2015. P. heparana catches decreased, while C. pomonella and H. nubiferana became more abundant (Fig. 1, Table S1). Catches were successfully shut down under MMD with numerical differences recorded for four species and no catches under mating disruption in the remaining three (Fig. 1). Adding R to MMD did not affect the catches. In the no MMD plots, no difference in catches was found between A and A + R (Fig. 1). Egg removal from cards ranged between 19 and 33% (Fig. 2). Significantly higher egg predation was measured in R compared to A with intermediate predation for MMD and A + R (Fig. 2, Table S1). The distance from the releasing device did not affect egg removal (GLMM: χ2 = 1.3, df = 3, P = 0.741, Table S1).
Mean proportion of predated egg cards (± SE) in 2016 and 2017. Bars with the same colour indicate the same experimental plot. Different letters indicate significant differences within the same year. Asterisk indicates statistically significant differences between years (GLMM: FDR-adjusted, P < 0.05). No letters indicate no differences between treatments within a given year. Multispecies mating disruption (MMD) treatment combinations (Fig. S1B) are not presented due to the null effect of the treatment on predators. The plot with MMD alone was regarded as control. R = Reward, A = Attract, + indicates the combination of treatments
Attract, reward and disrupt (2017)
The only abundant and analysed species caught in pheromone traps were H. nubiferana, S. ocellana and C. pomonella (Fig. 1). In 2017, egg predation ranged between 41 and 47% (Fig. 2). Although the highest predation was measured in the A + R treatment, there was no difference between treatments (Fig. 2, Table S1). A higher predation than in 2015 was observed in the A and A + R plots. Survival of sentinel aphid colonies was unaffected by the treatment (Frailty model: χ2 = 2.0, df = 3, P = 0.589, Fig. S3A) and no difference was found in natural enemies presence in aphid colonies over the 4 weeks of the experiment (Frailty model: χ2 = 4.7, df = 3, P = 0.237, Fig. S3B).
Establishment of flower strips
The most successful species in terms of establishment were Medicago lupulina, Lotus corniculatus, Achillea millefolium, Hypochaeris radicata, Galium mollugo, Cichorium intybus, Geranium pyrenaicum and Leucanthemum vulgare (Fig. S4). Species that did not establish included three grasses and four dicotyledons. Most of these species belong to the functional agrobiodiversity (FAB) group (Pfiffner et al. 2019), owing their positive properties towards beneficial insects. A high proportion of relevant FAB plants established in our orchards performing well under the evaluated period. Most species had their first flower in mid-June, while their flowering peak occurred at the beginning of August. Cutting off the flower strips could be adjusted to increase flowering around the oviposition period of tortricid moths. The most established sown grasses were Lolium perenne and Poa trivialis (Fig. S4). Flower strips were invaded by native non-grass species (Taraxacum sp., Trifolium pratense, Trifolium repens, Rumex crispus and Veronica arvensis), which in some cases were abundant (Fig. S4). However, the species community composition differed clearly between treatments including flower strips (A and A + R) and the other two (MMD and R) (Fig. S5 and S6).
Pest larval density in flower clusters
The highest leafroller larvae infestation at the end of the experiment (2018) was found in MMD + R, whilst the lowest in MMD + A (Fig. 3, LR). These treatments differed from each other. Although MMD + A + R and MMD had intermediate infestation, they did not differ from the other two (Fig. 3, Table S1). A significant increase in leafroller infestation occurred in time from 2015 to 2018 under the treatments MMD and the MMD + R, while no increase was found for the treatments A and the A + R (Fig. 3, LR, Table S1). Major species in 2018 were H. nubiferana (50.3% of the total larvae recorded) and S. ocellana (22.6%). A higher level of S. ocellana was measured in R compared to A in 2018, while leafroller larvae density decreased only in A over the 3 years (Fig. 3, So). When H. nubiferana density (not targeted by MMD) was subtracted from the total tortricid infestation level (Fig. 3, LR-Hn), there was no difference between treatments. The infestation level of H. nubiferana (Fig. 3, Hn) mirrored that of the total tortricids (Fig. 3, LR).
Average number (± SE) of leafrollers (LR), leafrollers excluding H. nubiferana (LR-Hn) (only present in 2018), H. nubiferana (Hn), S. ocellana (So) and geometrid larvae per flower cluster at BBCH 59 before any treatments were established (2015) and at the end of the study (2018). Bars with the same colour indicate the same experimental plot. Different letters indicate statistically significant differences within the same year and an asterisk indicates statistically significant higher abundance between years for a given treatment (GLMM: FDR-adjusted, P < 0.05). No letters indicate no differences between treatments within a given year. MMD = Multispecies mating disruption, R = Reward, A = Attract, + indicates the combination of treatments
A significant decrease in the geometrid population occurred from 2015 to 2018 in all plots (Fig. 3, Geometrids). The majority of the geometrids recorded belonged to the species Operophtera brumata (L.) (91.0%). In 2018, the lowest infestation was found for MMD + A + R, which did not differ from MMD + R. A higher level was measured for MMD + A, whilst the highest infestation was found in the MMD alone treatment (Fig. 3, Geometrids). Concerning larvae per flower cluster, the overall infestation decreased in all treatments, with MMD + A + R and MMD + R showing the highest reduction (Fig. 4a, Table S1). When considering the relative infestation decrease by caterpillars from 2015 to 2018, MMD + A + R and MMD + A scored a higher population decrease than the other two treatments (Fig. 4b).
a Average number (± SE) of feeding tortricid larvae per flower cluster before any treatments were established (2015) and at the end of the study 2018. b Relative decrease in infestation from 2015 to 2018. Bars with the same colour indicate the same experimental plot. Bars capped with the different letters in (A) differ significantly within the same year (GLMM: FDR-adjusted, P < 0.05). Bars capped with an asterisk in (A) significantly differed between years (GLMM: FDR-adjusted, P < 0.05). Bars capped with the different letters in (B) significantly differ from each other (GLMM: FDR-adjusted, P < 0.05). MMD = Multispecies mating disruption, R = Reward, A = Attract, + indicates the combination of treatments
Arthropod abundance in the canopy
Major predators collected were predatory mites, ladybirds (Coccinellidae), Anthocoris nemorum (L.) and Orius sp. (Anthocoridae), Atractotomus mali (Meyer), Heterotoma planicornis (Pallas), Psallus sp. (Miridae), Chrysoperla carnea s.l. (Chrysopidae), Forficula auricularia L. (Forficulidae), spiders (Araneae) and hoverflies (Syrphidae) (Table S2). Spiders were the most abundant group of predators, followed by earwigs, A. mali, H. planicornis, predatory mites and Anthocoridae (> 100 caught specimens). Platygastridae, Pteromalidae, Eulophidae and Braconidae were the parasitoid families sampled in highest numbers (> 100 caught specimens). Additional potential tortricid parasitoids included Ichneumonidae, Aphelinidae, Encyrtidae and Scelioninae (Table S2).
Whereas predatory insects (as a sum of Miridae, Anthocoridae, Cantharidae, Coccinellidae, Staphylinidae, Neuroptera, Dermaptera, and Formicidae) were more abundant in June in both 2016 and 2017, parasitoids (as a sum of Bethylidae, Ceraphronoidea, Cynipidae, Diapriidae, Ichneumonoidea, Platygastridae, Proctotrupidae, and Chalcidoidea) and spiders were more abundant in July in both years (Fig. S6). Overall, A + R hosted the highest density of natural enemies, whilst the control (MMD) and R scored the lowest level and A was located in the middle (Fig. 5, Table S1). Predator abundance was higher in A + R than in the control (MMD) and R, while the abundance in A was at an intermediate level between A + R and the other two variants. Parasitoids were less affected than predators by the tested treatments with no differences observed between them (Fig. 5). So was the case of spider abundance, also unaffected by the treatments.
Average number (± SE) of total natural enemies, predatory insects, parasitoids and spiders collected through suction sampling. Bars with the same colour indicate the same experimental treatment. Different letters indicate statistically significant differences between treatments (GLMM: FDR-adjusted, P < 0.05). No letters indicate no differences between treatments. The plot with MMD alone was regarded as control. R = Reward, A = Attract, + indicates the combination of treatments
Whereas the family Miridae (with A. mali and H. planicornis) was represented by high numbers and influenced by the treatments (Fig. 6, Table S1), Anthocoridae, including Orius sp. and A. nemorum, scored a lower number and were unaffected. Concerning predatory mirids, the A + R variant showed higher abundance than R and the control (MMD), while the levels of A and A + R did not differ from each other (Fig. 6). Both H. planicornis and A. mali showed the higher abundance in A + R (Fig. 6). The main parasitoid superfamilies collected, Ichneumonoidea and Chalcidoidea, showed no differences between treatments (Fig. 7, Table S1), however, the lower abundance levels were recorded in the control (MMD) for both.
Average number (± SE) of predatory Heteroptera collected through suction sampling. Bars with the same colour indicate the same experimental treatment. Different letters indicate statistically significant differences between treatments (GLMM: FDR-adjusted, P < 0.05). No letters indicate no differences between treatments. The plot with MMD alone was regarded as control. R = Reward, A = Attract, + indicates the combination of treatments
Average number (± SE) of Ichneumonidea and Chalcidoidea collected through suction sampling per plot. Bars with the same colour indicate the same experimental treatment. No statistically significant differences between treatments were detected (GLMM: FDR-adjusted, P > 0.05). The plot with MMD alone was regarded as the control. R = Reward, A = Attract, + indicates the combination of treatments
Discussion
Our simplified production landscapes have, together with a heavy reliance on pesticides, contributed to an unprecedented disruption of natural ecosystems and a collapse of ecosystem services and resilience (Hallmann et al. 2017). A sustainability overhaul is needed in order to re-establish a functional biodiversity in agroecosystem. However, rebuilding slow-responding ecosystems cannot be achieved by simple tweaks and fixes and requires intensive knowledge, and longer-term interventions (Tittonel 2014). These are difficult to implement in annual cropping systems but are more feasible in perennial crops. With rotation cycles of one to two decades, apple appears very amenable for studies on the effect of sustainable interventions on ecosystem diversity, services and resilience.
In our study in organic apple orchards, we demonstrate that deploying a complementary set of sustainable chemical ecological and habitat diversification tools correlated with increased population levels of natural enemies and lower levels of pest insects. This supports our initial hypothesis that simultaneously deploying semiochemicals and conservation biological methods increases biodiversity and the level of biological control. This is particularly encouraging since the observations were made in the relatively short experimental period, using a limited number of orchards, and in organic production settings, whose already enhanced biodiversity and biological control (e.g. Porcel et al. 2018) could have overshadowed the effects of our treatments. In addition, the baseline intervention method, MMD was rolled out in all plots and likely already suppressed tortricid population levels (Porcel et al. 2015).
We also note that this combination of tools has not been used before, although the effect of each individual component in suppressing pest populations has been reported before in various crops (Reward: Cahenzli et al. 2019; Attract: Pålsson et al. 2019; Disrupt: Porcel et al. 2015). In a limited number of studies, binary combinations of these tools have been tested. Synthetic HIPVs and buckwheat enriched natural enemy populations and biological control in broccoli, grapevine, sweetcorn (Simpson et al. 2011a, 2011b) and brassica (Gordon et al. 2013) although the authors indicated that potential synergies as well as unwanted effects required further research.
The overall combinatorial effects of the treatments support our hypothesis, nonetheless, zooming in to particular groups of pests or natural enemies showed considerable variation, some of which we highlight below. These variations are likely due to a number of factors that are outside our direct control and observations, but could be important when rolling out treatments in commercial orchards and should therefore be noted.
First, different from literature (e.g. see Winkler et al. 2009), we found no enhancement of aphids biological control by flower strips alone. In apple, pioneer work by Wyss (1995) demonstrated that 1–2 year-old perennial strips consisting of wild flowers increased the presence of natural enemies and biocontrol of D. plantaginea. Vogt and Weigel (1999) found that A. pomi was suppressed in flower strip-associated apple trees, but did not find a positive effect for D. plantaginea. The difference may be due to interannual variations that may blur correlations. In addition, a higher abundance of natural enemies may not automatically cascade to an increase in biocontrol. Also in our study the overall higher abundance of natural enemies did not decrease the survival of D. plantaginea colonies. This contrasts with an earlier study in which increased biological control of D. plantaginea was observed in organic compared to IPM orchards (Porcel et al. 2018). This disparity may be due to initial higher abundance of natural enemies in all organic orchards used in this study, which makes additive effects more difficult to achieve. Additionally, unlike Porcel et al. (2018), we did not exclude ants tending our sentinel colonies, which are known to strongly protect aphids from natural enemies. We suggest that aphids require additional intervention for sufficient suppression in organic orchards, as recently discussed by Pålsson et al. (2020).
Second, our R only treatment did not increase predator populations in the canopy. This may not be surprising, because flower strips do not always support very mobile natural enemies (Gurr et al. 2012; Herz et al. 2019). The build-up of beneficial arthropod populations is likely a long-term process, particularly when using perennial flower strips. In Canada, for example, 5 years were necessary to achieve a damage reduction (from 95.2 to 9.2% damage) from when flowers were introduced in orchards (Bostanian et al. 2004).
Third, lepidopteran larval and adult population levels varied considerably between species and treatments. The geometrid larval density decreased substantially from 2015 to 2018, but significantly more so in A + R and R than in MMD plots. The most abundant geometrid retrieved from clusters in 2018 was O. brumata, which pupates in the ground. It is possible that flower strips supported ground dwelling natural enemies in the R treatment, although this was not verified in our study. In contrast, the overall tortricid larval density slightly increased from 2015 to 2018 in the MMD and R, due to a rise of H. nubiferana and S. ocellana populations. Considering all tortricids except for H. nubiferana, a slight decrease occurred, with no difference among the four treatments. H. nubiferana populations thus increased likely because mating disruption affects other species to a higher extent than H. nubiferana (Porcel et al. 2015), and probably resulted in competitive release of the latter. Whereas the R component appears to benefit overwintering populations of H. nubiferana and S. ocellana, the A component probably counteracted this effect in both species. Some natural enemies may prefer to forage within the flower strips, which offers a higher prey level than the tree canopy. By adding the A component, natural enemies may be recruited to the tree instead of staying in the flower strip.
Finally, interannual variation was observed in egg predation between treatments. An increase in egg predation was measured for A and A + R from 2016 to 2017 in comparison to MMD and R. However, in spite of the increased egg removal in A + R, we found no correlation between egg removal by predators and predator density estimation through suction sampling. Similar findings were reported by Cahenzli et al. (2019), who found sentinel eggs removal unsuitable to reflect predator abundance.
The use of HIPVs to selectively recruit natural enemies has been investigated over the last two decades with variable results (Turlings and Erb 2018). In an earlier study we found that the three-component blend of AA, MS and PAA attracted mostly C. carnea s.l. besides Vespidae, and for over 4-weeks (Pålsson et al. 2019). In the present study, the HIPVs positively correlated with a suppression of caterpillars in flower clusters. Recruitment of natural enemies by this blend (besides C. carnea s.l.) has rarely been reported in the literature previously, although Simpson et al. (2011a; 2011b) reported attraction of predators and parasitoids with sprays containing MS. In comparison with sprayed HIPVs on crop vegetation (Simpson et al. 2011b), the dispensing device used in our study recruited significant numbers of natural enemies over a substantially longer period.
Predator recruitment using HIPVs benefited when combined with flower strips in the A + R plots. The combination of A + R enhanced the presence of Miridae which was mirrored by the lowest Lepidoptera infestation level in flower clusters toward the end of the study. A further confirmation of the positive influence of A + R on biological control comes from the proportion of sentinel eggs predated by native natural enemies, which showed a significant increase from 2016 to 2017.
The method we used to measure population density of natural enemies does not allow a precise estimation of the level of some species such as earwigs (active at night) and predatory mites. This is probably the reason why we did not detect any major influences of A, R or their combination on these important predators. We conclude that the use of synthetic odours to enhance natural enemy recruitment and ecosystem services should be further explored as an integral candidate for future management strategies.
In our study, we chose perennial over annual wildflowers as growers pointed out the importance of reducing costs of management. Perennial species established well along the 3-year period of the study in Southern Sweden, but may also have a slower impact than annual species whose effects are well documented (Gontijo et al. 2013). While the long-term performance needs further study, perennial flower strips significantly enriched the population of beneficial insects when combined with HIPVs and would appear an economically more sustainable option (Pfiffner et al. 2019).
Whereas our study is of practical relevance because it was carried out in commercial orchards, the sub-optimal distance between plots needs to be considered when evaluating our results. Although larger plot-plot distances are preferable and more common in studies of subsidiary vegetation in annual crops, we decided in favour of smaller plots for each treatment to be present in each orchard, ensuring similar environmental conditions and management practices across our four treatment plots, and thus reducing the risk of uncontrolled variation. Small plot size does not need to be problematic, as significant differences in natural enemy densities and biological control has been reported for even shorter plot–plot distances (Campbell et al. 2017; Simpson et al. 2011a). A possible drawback of our study is the change of treatments from 2016 to 2017. Because we complemented the A and the A + R treatments with MMD in 2017 in order to rule out a possible effect of MMD on population level of natural enemies, we cannot exclude an uncontrolled interference of such complementation on the herbivore/natural enemy balance.
Although promising, our results point towards the need of additional, ideally longer-term, research to fully assess the interactions between Attract, Reward and Disrupt. Besides the need for longer implementation times, satisfactory control of pests may need additional management practises. These may include better or more complex A blends (Simpson et al. 2011b; Campbell et al. 2017; Turlings and Erb 2018) than this lacewing-based attractant, or the use of the aforementioned diversion of ants to enhance control of aphids (Porcel et al. 2018). We strongly recommend continuation of research on such sustainable solutions to support rebuilding ecosystem services in commercial crops.
Restoration of ecosystem resilience requires time, and so do studies on the tools that support these transitions. Accordingly, long-term research programs are needed to understand the interaction of various sustainable interventions, such as Attract, Reward and Disrupt, in the context of surrounding landscape, orchard management and agroenvironmental schemes.
Author contributions
All authors conceived the ideas and designed the methodology. MT and MP procured funding. JP, MP and MT collected the data. JP and MP analysed the data. MT, JP and TD led the writing of the manuscript. All authors supervised JP as a part of his PhD education and contributed critically to the drafts and gave final approval for publication.
References
Bostanian NJ, Goulet H, O’Hara J, Masner L, Racette G (2004) Towards insecticide free apple orchards: flowering plants to attract beneficial arthropods. Biocontrol Sci Technol 14:25–37. https://doi.org/10.1080/09583150310001606570
Cahenzli F, Sigsgaard L, Daniel C, Herz A, Jamar L, Kelderer M, Kramer Jacobsen S, Kruczyńska D, Matray S, Porcel M, Sekrecka M, Swiergiel W, Tasin M, Telfser J, Pfiffner L (2019) Perennial flower strips for pest control in organic apple orchards: a pan-European study. Agric Ecosyst Environ 278:43–53. https://doi.org/10.1016/j.agee.2019.03.011
Campbell AJ, Wilby A, Sutton P, Wackers F (2017) Getting more power from your flowers: multi-functional flower strips enhance pollinators and pest control agents in apple orchards. Insects. https://doi.org/10.3390/insects8030101
Gontijo LM, Beers EH, Snyder WE (2013) Flowers promote aphid suppression in apple orchards. Biol Control 66:8–15. https://doi.org/10.1016/j.biocontrol.2013.03.007
Gordon G, Wratten SD, Jonsson M, Simpson M, Hale R (2013) “Attract and reward”: combining a herbivore-induced plant volatile with floral resource supplementation—multi-trophic level effects. Biol Control 64:106–115. https://doi.org/10.1016/j.biocontrol.2012.10.003
Gurr GM, Wratten SD, Barbosa P (2000) Success in conservation biological control of arthropods. In: Gurr G, Wratten S (eds) Biological control: measures of success. Springer, Dordrecht, pp 105–132
Gurr GM, Wratten SD, Snyder WE (2012) Biodiversity and insect pests: key issues for sustainable management. Wiley-Blackwell, Chichester
Hallmann CA, Sorg M, Jongejans E, Siepel H, Hofland N, Schwan H, Stenmans W, Müller A, Sumser H, Hörren T, Goulson D, de Kroon H (2017) More than 75 percent decline over 27 years in total flying insect biomass in protected areas. PLoS ONE 12:e0185809. https://doi.org/10.1371/journal.pone.0185809
Happe A, Alins G, Blüthgen N, Boreux V, Bosch J, García D, Hambäck P, Klein AM, Martínez-Sastre R, Miñarro M, Porcel M, Rodrigo A, Roquer-Beni L, Samnegård U, Tasin M, Mody K (2019) Predatory arthropods in apple orchards across Europe: responses to agricultural management, local habitats, landscape composition and country. Agric Ecosyst Environ 273:141–150. https://doi.org/10.1016/j.agee.2018.12.012
Herz A, Cahenzli F, Penvern S, Pfiffner L, Tasin M, Sigsgaard L (2019) Managing floral resources in apple orchards for pest control: ideas, experiences and future directions. Insects 10:247
Ioriatti C, Lucchi A (2016) Semiochemical strategies for tortricid moth control in apple orchards and vineyards in Italy. J Chem Ecol 42:571–583. https://doi.org/10.1007/s10886-016-0722-y
Orre GUS, Wratten SD, Jonsson M, Hale RJ (2010) Effects of an herbivore-induced plant volatile on arthropods from three trophic levels in brassicas. Biol Control 53:62–67. https://doi.org/10.1016/j.biocontrol.2009.10.010
Pålsson J, Thöming G, Silva R, Porcel M, Dekker T, Tasin M (2019) Recruiting on the spot: A biodegradable formulation for lacewings to trigger biological control of aphids. Insects 10:6. https://doi.org/10.3390/insects10010006
Pålsson J, Porcel M, Frimodt Hansen M, Offenberg J, Nardin T, Larcher R, Tasin M (2020) Aphid-infested beans divert ant attendance from the rosy apple aphid in apple-bean intercropping. Sci Rep 10(1):1–12. https://doi.org/10.1038/s41598-020-64973-7
Pfiffner L, Cahenzli F, Steinemann B, Jamar L, Chor Bjørn M, Porcel M, Tasin M, Telfser J, Kelderer M, Lisek J, Sigsgaard L (2019) Design, implementation and management of perennial flower strips to promote functional agrobiodiversity in organic apple orchards: a pan-European study. Agric Ecosyst Environ 278:61–71. https://doi.org/10.1016/j.agee.2019.03.005
Porcel M, Andersson G, Pålsson J, Tasin M (2018) Organic management in apple orchards: higher impacts on biological control than on pollination. J Appl Ecol 55:2779–2789. https://doi.org/10.1111/1365-2664.13247
Porcel M, Cotes B, Castro J, Campos M (2017) The effect of resident vegetation cover on abundance and diversity of green lacewings (Neuroptera: Chrysopidae) on olive trees. J Pest Sci 90:195–206. https://doi.org/10.1007/s10340-016-0748-5
Porcel M, Sjoberg P, Swiergiel W, Dinwiddie R, Ramert B, Tasin M (2015) Mating disruption of Spilonota ocellana and other apple orchard tortricids using a multispecies reservoir dispenser. Pest Manag Sci 71:562–570. https://doi.org/10.1002/ps.3844
R Development Core Team (2016) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna
Reganold JP, Glover JD, Andrews PK, Hinman HR (2001) Sustainability of three apple production systems. Nature 410:926–930. https://doi.org/10.1038/35073574
Rockstrom J, Williams J, Daily G, Noble A, Matthews N, Gordon L, Wetterstrand H, DeClerck F, Shah M, Steduto P, de Fraiture C, Hatibu N, Unver O, Bird J, Sibanda L, Smith J (2017) Sustainable intensification of agriculture for human prosperity and global sustainability. Ambio 46:4–17. https://doi.org/10.1007/s13280-016-0793-6
Rusch A, Chaplin-Kramer R, Gardiner MM, Hawro V, Holland J, Landis D, Thies C, Tscharntke T, Weisser WW, Winqvist C, Woltz M, Bommarco R (2016) Agricultural landscape simplification reduces natural pest control: a quantitative synthesis. Agric Ecosyst Environ 221:198–204. https://doi.org/10.1016/j.agee.2016.01.039
Samnegård U, Alins G, Boreux V, Bosch J, García D, Happe A, Klein A, Miñarro M, Mody K, Porcel M, Rodrigo A, Roquer-Beni L, Tasin M, Hambäck P (2019) Management trade-offs on ecosystem services in apple orchards across Europe: direct and indirect effects of organic farming. J Appl Ecol 56:802–811. https://doi.org/10.1111/1365-2664.13292
Simpson M, Gurr GM, Simmons AT, Wratten SD, James DG, Leeson G, Nicol HI, Orre GUS (2011a) Field evaluation of the “attract and reward” biological control approach in vineyards. Ann Appl Biol 159:69–78. https://doi.org/10.1111/j.1744-7348.2011.00477.x
Simpson M, Gurr GM, Simmons AT, Wratten SD, James DG, Leeson G, Nicol HI, Orre-Gordon GUS (2011b) Attract and reward: combining chemical ecology and habitat manipulation to enhance biological control in field crops. J Appl Ecol 48:580–590. https://doi.org/10.1111/j.1365-2664.2010.01946.x
Solomon M, Cross J, Fitzgerald J, Cambell C, Jolly R, Olszak R, Niemczyk E, Vogt H (2000) Biocontrol of pests of apples and pears in northern and central Europe. 3 predators. Biocontrol Sci Technol 10:91–128
Tittonell P (2014) Ecological intensification of agriculture: sustainable by nature. Curr Opin Environ Sustain 8:53–61. https://doi.org/10.1016/j.cosust.2014.08.006
Toth M, Szentkiralyi F, Vuts J, Letardi A, Tabilio MR, Jaastad G, Knudsen GK (2009) Optimization of a phenylacetaldehyde-based attractant for common green lacewings (Chrysoperla carnea s.l.). J Chem Ecol 35:449–458. https://doi.org/10.1007/s10886-009-9614-8
Tscharntke T, Klein AM, Kruess A, Steffan-Dewenter I, Thies C (2005) Landscape perspectives on agricultural intensification and biodiversity: ecosystem service management. Ecol Lett 8:857–874. https://doi.org/10.1111/j.1461-0248.2005.00782.x
Turlings TCJ, Erb M (2018) Tritrophic interactions mediated by herbivore-induced plant volatiles: mechanisms, ecological relevance, and application potential. Annu Rev Entomol 63:433–452. https://doi.org/10.1146/annurev-ento-020117-043507
Vogt H, Weigel A (1999) Is it possible to enhance the biological control of aphids in an apple orchard with flowering strips? IOBC wprs Bull 22:39–46
Wäckers FL, van Rijn PCJ, Heimpel GE (2008) Honeydew as a food source for natural enemies: making the best of a bad meal? Biol Control 45:176–184. https://doi.org/10.1016/j.biocontrol.2008.01.007
Wilkinson TK, Landis DA (2005) Habitat diversification in biological control: The role of plant resources. In: Wäckers FL, van Rijn PCJ, Bruin J (eds) Plant provided food and plant-carnivore mutualism. Cambridge University Press, Cambridge, pp 305–325
Winkler K, Wäckers FL, Kaufman LV, Larraz V, van Lenteren JC (2009) Nectar exploitation by herbivores and their parasitoids is a function of flower species and relative humidity. Biol Control 50:299–306
Wyss E (1995) The effects of weed strips on aphids and aphidophagous predators in an apple orchard. Entomol Exp Appl 75:43–49. https://doi.org/10.1111/j.1570-7458.1995.tb01908.x
Acknowledgements
We greatly acknowledge the growers who hosted our study and carefully followed our experimental protocol. This project was financed by The Swedish Research Council for Sustainable Development (Formas, project 2013-934) and the Swedish Agricultural Research Foundation grant (SLF R-18-25-016). We are thankful to the Eco-Orchard (Core Organic Plus) consortium for sharing knowledge about flower species. We are also thankful to Mona Chor Bjørn, Mette Frimodt Hansen, Giulia Attocchi, Emilia Muhlhäuser and Weronika Swiergiel for their help with fieldwork.
Funding
Open access funding provided by Swedish University of Agricultural Sciences.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Blas Lavandero.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Pålsson, J., Porcel, M., Dekker, T. et al. Attract, reward and disrupt: responses of pests and natural enemies to combinations of habitat manipulation and semiochemicals in organic apple. J Pest Sci 95, 619–631 (2022). https://doi.org/10.1007/s10340-021-01410-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10340-021-01410-2