Abstract
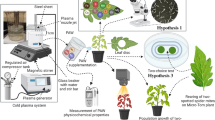
The use of systemic insecticides as seed treatments has raised concern about the possible impacts of these products on natural enemies. This study assessed the effects of sunflower seed treatments with chlorantraniliprole and thiamethoxam on Chrysoperla carnea by exposing larvae and adults to sunflower stem segments grown from treated seeds and the nectar secreted by their extrafloral nectaries. Confinement of larvae with stem segments for their entire developmental period had no effect on their survival or any life history parameter, except that the sex ratio of resulting adults was lower in the thiamethoxam treatment than in chlorantraniliprole. However, when adult pairs of C. carnea were exposed to treated stem segments during their maturation period, their subsequent survival and fecundity was significantly reduced by both materials, with thiamethoxam reducing median survival (LT50) and fecundity to a greater degree than chlorantraniliprole. Insufficient offspring were obtained from adults exposed to thiamethoxam to permit assessment of their fitness, but the offspring in the chlorantraniliprole-exposed adults had reduced larval survival relative to controls. The greater impact of seed treatments on adult lacewings may be partly attributable to their greater consumption of extra-floral nectar. Our results indicate that seed treatment with systemic insecticides can cause negative effects on beneficial insects, potentially disrupting their population dynamics, and should not be assumed compatible with biological control and IPM simply because this mode of application limits direct exposure.


Similar content being viewed by others
References
Albajes R, López C, Pons X (2003) Predatory fauna in cornfields and response to imidacloprid seed treatment. J Econ Entomol 96:1805–1813
Albuquerque GS, Tauber CA, Tauber MJ (1994) Chrysoperla externa (Neuroptera: Chrysopidae): life history and potential for biological control in Central and South America. Biol Control 4:8–13
Amarasekare KG, Shearer PW (2013) Comparing effects of Insecticides on two green lacewing species, Chrysoperla johnsoni and Chrysoperla carnea (Neuroptera: Chrysopidae). J Econ Entomol 106:1126–1133
Benzidane Y, Touinsi S, Motte E, Jadas-Hecart A, Communal PY, Leduc L, Thany SH (2010) Effect of thiamethoxam on cockroach locomotor activity is associated with its metabolite clothianidin. Pest Manag Sci 66:1351–1359
Biondi A, Mommaerts V, Smagghe G, Viñuela E, Zappalà L, Desneux N (2012a) The non-target impact of spinosyns on beneficial arthropods. Pest Manag Sci 68:1523–1536
Biondi A, Desneux N, Siscaro G, Zappalà L (2012b) Using organic-certified rather than synthetic pesticides may not be safer for biological control agents: selectivity and side effects of 14 pesticides on the predator Orius laevigatus. Chemosphere 87:803–812
Biondi A, Zappalà L, Stark JD, Desneux N (2013) Do biopesticides affect the demographic traits of a parasitoid wasp and its biocontrol services through sublethal effects? PlosOne 8(9):e76548
Bonmatin JM, Marchand PA, Charvet R, Moineau I, Bengsch ER, Colin AE (2005) Quantification of imidacloprid uptake in maize crops. J Agric Food Chem 53:5336–5341
Bradshaw JD, Rice ME, Hill JH (2008) Evaluation of management strategies for bean leaf beetles (Coleoptera: Chrysomelidae) and bean pod mottle virus (Comoviridae) in soybean. J Econ Entomol 101:1211–1227
Brewer MJ, Noma T, Elliott NC, Kravchenko AN, Hild AL (2008) A landscape view of cereal aphid parasitoid dynamics reveals sensitivity to farm- and region-scale vegetation structure. Eur J Entomol 105:503–511
Buchholz A, Nauen R (2002) Translocation and translaminar bioavailability of two neonicotinoid insecticides after foliar application to cabbage and cotton. Pest Manag Sci 58:10–16
Casida JE (2011) Neonicotinoid metabolism: compounds, substituents, pathways, enzymes, organisms, and relevance. J Agric Food Chem 59:2923–2931
Choate BA, Lundgren JG (2013) Why eat extrafloral nectar? Understanding food selection by Coleomegilla maculata (Coleoptera: Coccinellidae). Biocontrol 58:359–367
Cloyd RA, Bethke JA (2011) Impact of neonicotinoid insecticides on natural enemies in greenhouse and interiorscape environments. Pest Manag Sci 67:3–9
Cutler GC, Scott-Dupree CD (2007) Exposure to clothianidin seed-treated canola has no long-term impact on honey bees. J Econ Entomol 100:765–772
Desneux N, Decourtye A, Delpuech JM (2007) The sublethal effects of pesticides on beneficial organisms. Ann Rev Entomol 52:81–106
Dively GP, Kamel A (2012) Insecticide residues in pollen and nectar of a cucurbit crop and their potential exposure to pollinators. J Agric Food Chem 60:4449–4456
Downes JA (1974) Sugar feeding by larva of Chrysopa (Neuroptera). Can Entomol 106:121–125
Easton AH, Goulson D (2013) The neonicotinoid insecticide imidacloprid repels pollinating flies and beetles at field-realistic concentrations. PLoS ONE 8:e54819
Gontijo PC, Moscardini VF, Michaud JP, Carvalho GA (2014) Non-target effects of two sunflower seed treatments on Orius insidiosus (Hemiptera: Anthocoridae). Pest Manag Sci. doi:10.1002/ps.3798
Goulson D (2013) REVIEW: An overview of the environmental risks posed by neonicotinoid insecticides. J Appl Ecol 50:977–987
Gurr GM, Scarratt SL, Wratten SD, Berndt L, Irvin NA (2004) Ecological engineering, habitat manipulation and pest management. In: Gurr GM, Wratten SD, Altieri MA (eds) Ecological engineering for pest management. Comstock Press, Ithaca, pp 1–12
Hagen KS (1986) Ecosystem analysis: plant cultivars (HPR), entomophagous species and food supplements. In: Boethel DJ, Eikenbarrey RD (eds) Interactions of plant resistance and prasitoids and predators of insects. Wiley, New York, pp 151–197
He YX, Zhao JW, Zheng Y, Desneux N, Wu K (2012) Lethal effect of imidacloprid on the coccinellid predator Serangium japonicum and sublethal effects on predator voracity and on functional response to the whitefly Bemisia tabaci. Ecotoxicology 21:1291–1300
Hull L, Beers E (1985) Ecological selectivity: modifying chemical control practices to preserve natural enemies. In: Hoy MA, Herzog DC (eds) Biological control in agricultural IPM systems. Academic Press, New York, pp 103–122
Institute SAS (2008) SAS for Windows Version 90 SAS. Institute Cary, North Carolina
Krischik VA, Landmark AL, Heimpel GE (2007) Soil-applied imidacloprid is translocated to nectar and kills nectar-feeding Anagyrus pseudococci (Girault) (Hymenoptera: Encyrtidae). Environ Entomol 36:1238–1245
Krupke CH, Hunt GJ, Eitzer BD, Andino G, Given K (2012) Multiple routes of pesticide exposure for honey bees living near agricultural fields. PLoS ONE 7:e29268
Lahm GP, Stevenson TM, Selby TP, Freudenberger JH, Cordova D, Flexner L, Bellin CA, Dubas CM, Smith BK, Hughes KA, Hollingshaus JG, Clark CE, Benner EA (2007) Rynaxypyr: a new insecticidal anthranilic diamide that acts as a potent and selective ryanodine receptor activator. Bioorgan Med Chem Lett 17:6274–6279
Lahm GP, Cordova D, Barry JD (2009) New and selective ryanodine receptor activators for insect control. Bioorgan Med Chem 17:4127–4133
Lanka SK, Stout MJ, Beuzelin JM, Ottea JA (2014) Activity of chlorantraniliprole and thiamethoxam seed treatments on life stages of the rice water weevil as affected by the distribution of insecticides in rice plants. Pest Manag Sci 70:338–344
Laurent FM, Rathahao E (2003) Distribution of C-14 imidacloprid in sunflowers (Helianthus annuus L) following seed treatment. J Agr Food Chem 51:8005–8010
Li X, Degain BA, Harpold VS, Marcon PG, Nichols RL, Fournier AJ, Naranjo SE, Palumbo JC, Ellsworth PC (2012) Baseline susceptibilities of B- and Q-biotype Bemisia tabaci to anthranilic diamides in Arizona. Pest Manag Sci 68:83–91
Limburg DD, Rosenheim JA (2001) Extrafloral nectar consumption and its influence on survival and development of an omnivorous predator, larval Chrysoperla plorabunda (Neuroptera: Chrysopidae). Environ Entomol 30:595–604
Lundgren JG (2009) Relationships of natural enemies and non-prey foods. Springer International, Dordrecht
Martinou AF, Seraphides N, Stavrinides MC (2014) Lethal and behavioral effects of pesticides on the insect predator Macrolophus pygmaeus. Chemosphere 96:167–173
Michaud JP, Qureshi JA (2006) Reproductive diapause in Hippodamia convergens (Coleoptera: Coccinellidae) and its life history consequences. Biol Control 39:193–200
Moscardini VF, Gontijo PC, Michaud JP, Carvalho GA (2014) Sublethal effects of chlorantraniliprole and thiamethoxam seed treatments when Lysiphlebus testaceipes feed on sunflower extrafloral nectar. Biocontrol. doi:10.1007/s10526-014-9588-5
Nauen R, Reckmann U, Armborst S, Stupp HP, Elbert A (1999) Whitefly-active metabolites of imidacloprid: biological efficacy and translocation in cotton plants. Pestic Sci 55:265–271
Nauen R, Ebbinghaus-Kintscher U, Salgado VL, Kaussmann M (2003) Thiamethoxam is a neonicotinoid precursor converted to clothianidin in insects and plants. Pestic Biochem Phys 76:55–69
New TR (1975) The biology of Chrysopidae and Hemerobiidae (Neuroptera), with reference to their usage as biocontrol agents: a review. Trans R Entomol Soc Lond 127:115–140
Ohkawara Y, Akayama A, Matsuda K, Andersch W (2002) Clothianidin: a novel broad spectrum neonicotinoid insecticide. In: Proc Brighton Crop Protection Conference (ed) Pests and Diseases. Brighton, UK, pp 51–58
Pappas ML, Broufas GD, Koveos DS (2011) Chrysopid predators and their role in biological control. J Entomol 8:301–326
Patt JM, Wainright SC, Hamilton GC, Whittinghill D, Bosley K, Dietrick J, Lashomb JH (2003) Assimilation of carbon and nitrogen from pollen and nectar by a predaceous larva and its effects on growth and development. Ecol Entomol 28:717–728
Planes L, Catalán J, Tena A, Porcuna JL, Jacas JA, Izquierdo J, Urbaneja A (2013) Lethal and sublethal effects of spirotetramat on the mealybug destroyer, Cryptolaemus montrouzieri. J P Sci 86:321–327
Principi MM, Canard M (1984) Feeding habits. In: Canard M, Semeria Y, New TR (ed) Biology of Chrysopidae. The Hague, pp 76–92
Rogers MA, Krischik VA, Martin LA (2007) Effect of soil application of imidacloprid on survival of adult green lacewing, Chrysoperla carnea (Neuroptera: Chrysopidae), used for biological control in greenhouse. Biol Control 42:172–177
Royer TA, Walgenbach DD (1991) Predacious arthropods of cultivated sunflower in eastern South Dakota. J Kans Entomol Soc 64:112–116
Sanchez-Bayo F, Tennekes HA, Goka K (2013) Impact of systemic insecticides on organisms and ecosystems. In: Trdan S (ed) Insecticides - development of safer and more effective technologies. InTech, Croatia, pp 365–414
Schwarz M, Christie D, Andersch W, Kemper K, Fellmann K, Altmann R (2002) Control of corn rootworms (Diabrotica spp) and of secondary pests of corn (Zea mays) using seed treatment of clothianidin. In: Proc Brighton Crop Protection Conference (ed) Pests and Diseases. Brighton, UK, pp 59–64
Seagraves MP, Lundgren JG (2012) Effects of neonicitinoid seed treatments on soybean aphid and its natural enemies. J Pest Sci 85:125–132
Smagghe G, Deknopper J, Meeus I, Mommaerts V (2013) Dietary chlorantraniliprole suppresses reproduction in worker bumblebees. Pest Manag Sci 69:787–791
Stelzla M, Devetak D (1999) Neuroptera in agricultural ecosystems. Agric Ecosyst Environ 74:305–321
Stoner KA, Eitzer BD (2012) Movement of soil-applied imidacloprid and thiamethoxam into nectar and pollen of squash (Cucurbita pepo). PLoS ONE 7:e39114
Strausbaugh CA, Eujayl IA, Foote P (2010) Seed treatments for the control of insects and diseases in sugarbeet. J Sug Beet Res 47:105–125
Tomizawa M, Casida JE (2005) Neonicotinoid insecticide toxicology: mechanisms of selective action. Annu Rev Pharmacol 45:247–268
Venzon M, Rosado MC, Euzébio DE, Souza B, Schoereder JH (2006) Suitability of leguminous cover crop pollens as food source for the green lacewing Chrysoperla externa (Hagen) (Neuroptera: Chrysopidae). Neotrop Entomol 35:371–376
Wackers FL, Romeis J, van Rijn P (2007) Nectar and pollen feeding by insect herbivores and implications for multitrophic interactions. Annu Rev Entomol 52:301–323
Zotti JM, Grutzmacher AD, Lopes IH, Smagghe G (2013) Comparative effects of insecticides with different mechanisms of action on Chrysoperla externa (Neuroptera: Chrysopidae): lethal, sublethal and dose-response effects. Insect Sci 20:743–752
Acknowledgments
The authors are grateful to the CAPES Foundation (Brazilian Ministry of Education), the National Council of Scientific and Technological Development (CNPq), and the Minas Gerais State Foundation for Research Aid (FAPEMIG) for scholarship support from CAPES—no 3363-13-9 (PCG) and CAPES—no 3362-13-2 (VFM). This is contribution no. 14-294 of the Kansas State Experiment Station.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by A. Biondi.
Rights and permissions
About this article
Cite this article
Gontijo, P.C., Moscardini, V.F., Michaud, J.P. et al. Non-target effects of chlorantraniliprole and thiamethoxam on Chrysoperla carnea when employed as sunflower seed treatments. J Pest Sci 87, 711–719 (2014). https://doi.org/10.1007/s10340-014-0611-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10340-014-0611-5