Abstract
A novel capillary electrophoresis method was developed for the determination of new 4-aryl-pyrido[1,2-c]pyrimidine derivatives, potential antidepressant agents, in serum. The derivatives have conformationally restricted tryptamine moiety in pharmacophore portion and exhibit high affinity to molecular targets: 5-HT1A receptor and serotonin transporter protein. The separation process was conducted using an eCAP fused-silica capillary, detection wavelength 214 nm, 200 mM phosphate buffer adjusted to pH = 8.0, temperature 20 °C, voltage 5 kV. The proposed method was validated by determining its linearity in the concentration range of 200–1000 ng/mL. A satisfactory linearity was obtained for the method, with R2 from 0.9978 to 0.9999 for all five derivatives and a limit of quantification level from 287.1 to 310.1 ng/mL. The recoveries for all derivatives were in the range from 94.7 to 100%. The speed of obtaining the result of the analysis was only 3 min. The developed method allows to determine all five derivatives both in water solutions and serum.
Similar content being viewed by others
Introduction
Serotonin (5-HT) is a monoamine neurotransmitter in the human brain that is involved in the regulation of certain physiological functions, including the sleep–wake cycle, body temperature, blood pressure, and pain perception. It also regulates the extremely important hormonal functions of the hypothalamus and psychological functions such as depression and anxiety [1,2,3]. Therefore, maintaining an appropriate level of serotonin seems to play an important role in mood regulation, and thus, alleviating or eliminating depressive episodes.
Depressive problems associated with abnormal levels of serotonin can be conditioned by many factors such as defective synthesis, release, re-uptake and breakdown of 5-hydroxytryptamine, or a defect in one or more serotonergic receptors [4, 5]. In cases of confirmed serotonin deficiency, widely used pharmacological therapy is practiced, involving the administration of drugs from the group of selective serotonin reuptake inhibitors (SSRIs), which include citalopram, escitalopram, fluoxetine, fluvoxamine, paroxetine, and sertraline [6,7,8,9,10]. The mechanism of action of these drugs involves inhibiting the reabsorption of previously secreted serotonin into the presynaptic space, thanks to which its concentration increases between neuronal connections and the time of interaction of this neurotransmitter with serotonergic receptors on the surface of the postsynaptic membrane increases [11,12,13,14].
The second point of action of drugs from the group of SSRIs is the transmembrane serotonin transporter (SERT). This protein belongs to the soluble carriers of solute carrier 6 (SLC6) genes. This is connected with the activity of ATPase, also known as the sodium pump, which, by pumping sodium ions out of the cell, creates a concentration gradient that allows the transport of serotonin into the cell. The serotonin transporter has a specific binding site for this neurotransmitter and an allosteric site to which the drug binds. Blocking this protein significantly reduces its affinity for the substrate, and thus prevents the resorption of serotonin into the cell [15].
Considering the above, it seems extremely important to search for new antidepressants and to develop new methods of their investigations. The new antidepressant compounds (I, II, III, IV and V) investigated in this work are the recently synthesized 4-aryl-pyrido[1,2-c]pyrimidine derivatives (Fig. 1) [16]. Their action is associated with both high affinity to the 5-HT1A receptor and to the serotonin transporter protein [16], which makes these compounds very interesting from the point of view of their dual mechanism of action. Since the above mentioned derivatives (I, II, III, IV and V) will be the subject of further research, there is a real and great need to develop methods for their studies. The existence of a proven method that allows qualitative and quantitative research of new derivatives in biological material also seems to be very important in a case of its use in clinical trials. In turn, the study of their individual forms in aqueous solutions will be the basis for qualitative and quantitative measurements of these substances in various drug forms. Therefore, to meet the expectations, in this work a new method of capillary electrophoresis was developed for the determination of all five derivatives (I, II, III, IV and V) in aqueous solutions and biological material (serum). The aim of our research was to develop a fast and reproducible analytical method that would provide an appropriate limit of quantification (LOQ). Of course, capillary electrophoresis has been used in the past for the determination of other antidepressants [17,18,19] and many other active pharmaceutical ingredients [20,21,22,23,24,25,26,27,28], but to the best of our knowledge there are no reports of the determination of 4-aryl-pyrido[1,2-c]pyrimidine using capillary electrophoresis (CE). In the available literature many different pyrido[1,2]pyrimidine derivatives have been described, however, no work has described their clinical trials and thus their potential range of concentrations in serum and other body fluids [29,30,31,32,33,34,35,36,37].
Since the compounds presented in this work are currently in the early phase of preclinical research, we are not able to say at this time what their effective therapeutic concentration ranges in body fluids are. Therefore, this work shows the possibilities of determining the tested compounds in serum within the concentration ranges of the developed method. The lack of knowledge regarding the actual concentration values of the tested derivatives in the serum of potential patients may be (although not necessarily) the only limitation of the developed method in relation to the limit of quantitation of the tested derivatives. Nevertheless, this is the first work describing the possibilities of determining 4-aryl-pyrido[1,2-c]pyrimidine derivatives in serum, which may become the basis for the development of other techniques and methods for their testing.
Materials and Methods
Chemicals and Reagents
The synthesis and spectroscopic analysis nuclear magnetic resonance (NMR) and high resolution electrospray ionization mass spectrometry (ESI-HRMS) and the biological tests of the compounds have been described in the previous work [16]. The tested derivatives were synthesized and obtained from the laboratory of the Medical University of Warsaw. Purity of the reference compounds were found to be greater than 95%. All the other analytes applied in the separations had a purity of 99%. Sodium monophosphate, deionized water were purchased from Sigma-Aldrich (Darmstadt, Germany), ethyl acetate and methanol from Sigma-Aldrich (Steinheim, Germany), and hexane from Sigma-Aldrich. Human serum standard, was purchased from Sigma-Aldrich (Poznań, Poland). Paroxetine as an internal standard (IS) (Fig. 1) was purchased from Sigma-Aldrich (Poznań, Poland).
Instrumentation
Analysis was performed with Beckman P/ACE System MDQ capillary electrophoresis, equipped with an autosampler and a UV/Visible detector, was used. All the parameters of the CE were controlled by Karat software version 32. An eCAP fused-silica capillary (30 cm total length, 20 cm effective length, 50 µm id, 375 µm od) was used.
Sample Preparations
In the extraction procedure 500 µL of serum standard, 50 µL of each of the five derivatives I, II, III, IV and V at a concentration of 20 µg/mL, 50 µL of methanolic paroxetine solution (IS) at a concentration of 10 µg/mL and 3 mL of n-hexane/ethyl acetate (90:10, v/v) were added and shaken for 3 min. After centrifugation (5 min, 3000 rpm) of the layers, the organic layer was decanted and evaporated under a stream of nitrogen at 37 °C. The sample was dissolved in 100 µL of methanol: deionized water (1:1) and injected into the capillary.
CE Conditions
Electrophoretic separations were obtained using an eCAP fused silica capillary (30 cm length, 25 cm effective length, 50 μm I.D.). The compounds I, II, III, IV and V were determined using 200 mM sodium phosphate buffer as a background electrolyte (BGE) adjusted to pH = 8.0. Buffer before introduction into the capillary was filtered through a 0.45 μm pore size filter. Serum samples after extraction of n-hexane–ethyl acetate mixture in the ratio of 90:10 (v/v) and reconstitution in the 0.1 mL methanol:water solution (1:1) were automatically injected using pressure injection (20.685 Pa, 5 s). The vials containing the anode and cathode buffer were emptied and refilled with the running buffer before each analysis. The detector was set at 214 nm and the separation of compounds I, II, III, IV and V was carried out at a temperature of 20 °C and voltage of 5 kV. The separation processes were conducted for serum and water solutions (the results have been presented in the figures in the “Results and Discussion” section).
Preparation of Stock and Working Standard Solutions
Primary standard stock solutions for compounds I, II, III, IV and V were prepared separately in methanol (Sigma–Aldrich) with concentrations of 1 mg/mL, respectively. These solutions were further diluted with methanol: deionized water (1:1) to obtain mixed working standard solutions of 100, 200, 300, 400, 500, 700, and 1000 ng/mL. for derivatives I, II, III, IV and V respectively. The studies showed that all analytes were stable in solution. The prepared solutions were stored in a refrigerator at 3 °C and were stable for 30 days of research.
Results and Discussion
Method Development
In order to develop a method for the quantification of compounds I, II, III, IV and V by capillary electrophoresis, optimization of the method was carried out at different pH values and BGE concentrations. A buffer containing sodium monophosphate at the concentration of 200 mM adjusted to pH 8.0 with 0.1 M sodium hydroxide turned out to be the best separation system.
The effect of pH was studied in the range of 3.0–8.0 for the BGE. When the pH was 3.0 the peaks shape were inadequate and resolution for all investigated compounds I, II, III, IV and V was not obtained at this pH value (Fig. 2A). Therefore, by varying the pH, the best resolution was attempted. It turned out that, the resolution of the investigated compounds increased with increasing pH of the buffer. A clear but only partial separation of compounds I, II, III, IV and V was obtained at a buffer pH value of 6.0 (Fig. 2B). However the best results in terms of separation efficiency and analysis time were obtained using a pH of 8.0 (Fig. 2C). Investigations for the determination of the mentioned compounds were carried out in the range of buffer concentrations from 50 to 200 mM at pH 8.0. It was observed that good resolution could not be obtained at buffer concentrations lower than 50 mM (Fig. 2A). Only above the concentration of 50 mM the resolution increased with its concentration, which was clearly visible when the buffer concentration reached 100 mM (Fig. 2B). Finally, the best resolution was obtained with a buffer concentration of 200 mM (Fig. 2C). Resolution factors for adjacent peaks were also calculated. For the parameters presented in Fig. 2B, they were, respectively: Rs1 = 0.196, Rs2 = 0.109, Rs3 = 0.077, Rs4 = 0.066. However, for the parameters presented in Fig. 2C they were, respectively: Rs1 = 0.323, Rs2 = 0.200, Rs3 = 0.205, Rs4 = 0.104, which clearly indicates an increase in resolution. The migration times of adjacent peaks (t m1, t m2) and the width of these peaks base (W b1, W b2) were used to calculate the resolution. According to the formula: Rs = 2(tm2 − tm1)/(Wb1 + Wb2). Calculations have been performed for each pair of peaks.
Electropherograms: A separation of compounds I (1), II (2), III (3), IV (4) and V (5) at a concentration of 1000 ng/mL at pH = 3.0 and buffer concentration = 50 mM, B separation of compounds I (1), II (2), III (3), IV (4) and V (5) at a concentration of 1000 ng/mL at pH = 6.0 and buffer concentration = 100 mM. C separation of compounds I (1), II (2), III (3), IV (4) and V (5) at pH = 8.0 and buffer concentration = 200 mM
Different analytical conditions were also examined. Voltage, analysis temperature, capillary length, and detection wavelength were optimized in the ranges of 5–30 kV, 15–30 °C, 30–80 cm (total length), and 200–300 nm, respectively. Voltage was set to 5 kV, capillary temperature + 20 °C, capillary length was 30 cm and 50 μm I.D, and detection wavelengths was 214 nm. With optimized electrophoretic condition, the average migration times were 2.15 ± 0.023, 2.32 ± 0.027, 2.61 ± 0.026, 2.83 ± 0.024, and 3.16 ± 0.026 min, for I, II, IV and V respectively (Fig. 3A). The developed method has also been used to measure the compounds I, II, III, IV and V concentrations in serum (Fig. 3B). Based on the obtained results it was found that the proposed method allows the measure of the concentrations of these drugs using the extraction method.
Electropherograms: A separation of compounds I (1), II (2), III (3), IV (4) and V (5) at a concentration of 1000 ng/mL in water solution in the presence of an internal standard concentration of 500 ng/mL. B: separation of compounds I (1), II (2), III (3), IV (4) and V (5) at a concentration of 1000 ng/mL in the presence of an internal standard concentration of 500 ng/mL. in serum after extraction procedure. C blank serum after extraction procedure
Method Validation
The methodology for analysis of compounds I, II, III, IV and V using CE was validated in terms of selectivity, linearity, sensitivity, precision, and accuracy according to the FDA guidelines [38] and ICH guidelines [39].
Specificity
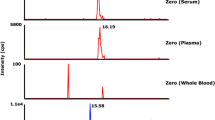
The selectivity of the method was evaluated by monitoring and comparing the quantification derivatives I, II, III, IV and V of each analyte in blank human plasma from six different sources to those in blank human plasma spiked with analytes at LOQ to check the possible interference at the peak regions. Selectivity was guaranteed if the interference due to endogenous substances was < 20% and < 5% of the mean peak response of each analyte at LOQ. The specificity of the method was confirmed by overlaying electropherograms of the blank solution and a sample of compound I, II, III, IV and V. An electropherogram obtained from a blank serum sample is represented in Fig. 3C. There were no interfering peaks of endogenous compounds observed at the retention time of the analytes.
Linearity, Precision and Sensitivity of the Method
Linearity
Six calibration curves were prepared using six calibrabtion points in the range of 200–1000 ng/mL for compounds I, II, III, IV and V, on six separate days. Calibration curves were constructed by plotting peak area ratios (analyte) versus nominal serum concentrations. Standard solutions of analytes I, II, III, IV and V were added to 0.5 mL of serum to produce final concentrations of 200, 300, 400, 500, 700, and 1000 ng/mL. The peak area values obtained for each sample were determined from the ratio of the analyte peak area/IS versus the analyte concentrations with respect to the six-point calibration curves. The correlation coefficients of calibration curves were in the range of 0.9987–0.9999 for all five compounds (Table 1).
Limit of Detection and Quantification
The limits of detection (LOD) and limits of quantification (LOQ) were determined in accordance with the FDA procedure as: LOD = 3.3 × δ/S and LOQ = 10 × δ/S (where δ = standard deviation and S = slope). The LOD was determined by analyzing loaded serum samples with increasing analyte content. Each concentration was analyzed six times. The proposed method allows the compounds I, II, III, IV and V to be determined with LODs between 50.6–58.5 ng/mL and LOQs between 167.1–186.1 ng/mL (Table 1).
Absolute Recovery
Analytical recoveries were performed at three different concentrations (300, 500, 1000 ng mL) for all five compounds in the presence of a constant internal standard concentration of 500 ng/mL. The recovery of the extraction procedure was determined by comparing the values of peak areas obtained with those of standard solutions of equivalent concentrations of five the analytes. The recovery for the 4-aryl-pyrido[1,2-c]pyrimidine derivatives was obtained by determining the ratio of the peaks areas of five 4-aryl-pyrido[1,2-c]pyrimidine derivatives to the peaks areas of the internal standard (paroxetine) against known amounts of (I), (II), (III), (IV) and (V) 4-aryl-pyrido[1,2-c]pyrimidine derivatives added to the plasma samples.. The recovery of concerned analytes was found in a satisfactory range of 95.5–100.0% (Table 2).
Intra and Inter-day Precision
Precision and accuracy of the method was carried out by analyzing the standard solutions of five 4-aryl-pyrido[1,2-c]pyrimidine derivatives at three different concentration levels (300.0 ng/mL, 500 ng/mL and 1000 ng/mL.) containing IS at a constant concentration of 500 ng/mL. (Table 3). The intra-day precision was within limits 2.2–5.3% RSD for the peak area. The inter-day precision was within limits 2.8–5.5% RSD for the peak area for all five investigated compounds. The precision results (Table 3) showed the low values of intra- and inter-day % RSD of peak areas (< 5.6%).
Conclusions
In this work, it has been shown that the developed CE method allows to determine new derivatives of 4-aryl-pyrido[1,2-c]pyrimidine in serum. A simple extraction method allowed the isolation of all investigated compounds from biological material. Based on the results, it can be concluded that the extraction method had good linearity for all tested compounds (R2 > 0.9978) According to available literature, the developed CE method has not yet been used to isolate and determine these kinds of antidepressant compounds in serum. The method was found to be suitable for determining the I, II, III, IV and V derivatives within the LOQ range 167.1–186.1 ng/mL of nominal analyte concentration. In addition, the RSD for intra- as well as inter-day precision was also low and did not exceed 6% for all investigated compounds.The reliability of the electrophoretic procedure for its desired application has been demonstrated by means of the electrophoretic procedure for its desired application by means of the experimental results like linearity, accuracy, specificity, sensitivity and precision. The presented results show that the CE method allowed fast and reliable screening and identification as well as accurate, precise, and sensitive quantification of compounds I, II, III, IV and V in serum.
Data Availability
Not applicable.
References
Owens MJ, Nemeroff CB (1994) Role of serotonin in the pathophysiology of depression: focus on the serotonin transporter. Clin Chem 40:288–295
Berger M, Gray JA, Roth BL (2009) The expanded biology of serotonin. Annu Rev Med 60:355–366. https://doi.org/10.1146/annurev.med.60.042307.110802
Jones LA, Sun EW, Martin AM, Keating DJ (2020) The ever-changing roles of serotonin. Int J Biochem Cell Biol 125:105776
Blier P, Mansari M (2013) Serotonin and beyond: therapeutics for major depression. Philos Trans R Soc Lond, B Biol Sci 368:20120536
Marazziti D, Landi P, Baroni S et al (2013) The role of platelet/lymphocyte serotonin transporter in depression and beyond. Curr Drug Targets 14(5):522–530
Brent Richards J, Papaioannou A, Adachi JD, Joseph L, Whits HE, Prior JC, Goltzman D (2007) Effect of selective serotonin reuptake inhibitors on the risk of fracture. Arch Intern Med 167:188–194
Lochmann D, Richardson T (2018) Selective serotonin reuptake inhibitors. In: Macaluso M, Preskorn S (eds) Antidepressants, handbook of experimental pharmacology, vol 250. Springer, Cham, pp 135–144
Carrasco JL, Sandner C (2005) Clinical effects of pharmacological variations in selective serotonin reuptake inhibitors: an overview. Int J Clin Pract 59(12):1428–1434
Fitzgerald TK, Bronstein AC (2013) Selective serotonin reuptake inhibitor exposure. Top Comp Anim Med 28:13–17
Taylor MJ, Freemantle N, Geddes JR, Bhagwagar Z (2006) Early onset of selective serotonin reuptake inhibitor antidepressant action, systematic review and meta-analysis. Arch Gen Psychiatry 63(11):1217–1223
Nutt DJ, Forshall S, Bell C, Rich A, Sandford J, Nash J, Argyropoulos S (1993) Mechanisms of action of selective serotonin reuptake inhibitors in the treatment of psychiatric disorders. Eur Neuropsychopharmacol 9(3):S81–S86
Stahl SM (1998) Mechanism of action of serotonin selective reuptake inhibitors: Serotonin receptors and pathways mediate therapeutic effects and side effects. J Affect Disord 51(3):215–235
Artigas F, Nutt DJ, Shelton R (2002) Mechanism of action of antidepressants. Psychopharmacol Bull 36(2):123–132
Fasipe OJ (2018) Neuropharmacological classification of antidepressant agents based on their mechanisms of action. Arch Med Health Sci 6(1):81–94
Rudnick G, Sandtner W (2019) Serotonin transport in the 21st century. J Gen Physiol 151(11):1248–1264
Ślifirski G, Król M, Kleps J, Ulenberg S, Belka M, Bączek T, Siwek A, Stachowicz K, Szewczyk B, Nowak G, Bojarski A, Kozioł AE, Turło J, Herold F (2019) Synthesis of novel pyrido[1,2-c]pyrimidine derivatives with rigidized tryptamine moiety as potential SSRI and 5-HT1A receptor ligands. Eur J Med Chem 166:144–158
Caixia Y, Hongwei D, Tianyan Y (2011) Determination of imipramine and trimipramine by capillary electrophoresis with electrochemiluminescence detection. Talanta 83(5):1376–1380. https://doi.org/10.1016/j.talanta.2010.11.011
Mandrioli R, Pucci V, Visini D, Varani G, Raggi MA (2002) Rapid methods for determination of fluoxetine in pharmaceutical formulations. J Pharm Biomed Anal 29:1127–1134
Fanali S, Cotichini V, Porrà R (1997) Analysis of venlafaxine by capillary zone electrophoresis. J Capill Electrophor 4(1):21–26
Amézqueta S, Subirats X, Fuguet E, Ràfols C, Rosés M (2020) Capillary electrophoresis for drug analysis and physicochemical characterization. In: Valkó KL (ed) Handbook of analytical separations, vol 8. Elsevier Science, pp 633–666
Shah M, Patel N, Tripathi N, Vyas VK (2022) Capillary electrophoresis methods for impurity profiling of drugs: a review of the past decade. J Pharm Anal 12(1):15–28. https://doi.org/10.1016/j.jpha.2021.06.009
Štěpánová S, Kašička V (2014) Determination of impurities and counterions of pharmaceuticals by capillary electromigration methods. J Sep Sci 37:2039–2055
Muhandiramge R, Quirino JP (2021) Sample preparation in capillary electrophoresis for the determination of small molecule drugs and metabolites in urine. Bioanalysis 13(7):533–536
Espada A, Molina-Martin M (2012) Capillary electrophoresis and small molecule drug discovery: a perfect match? Drug Discov Today 17:396–404
Tobolkina E, Rudaz S (2021) Capillary electrophoresis instruments for medical applications and falsified drug analysis/quality control in developing countries. Anal Chem 93:8107–8115. https://doi.org/10.1021/acs.analchem.1c00839
Colombo R, Papetti A (2019) Advances in the analysis of veterinary drug residues in food matrices by capillary electrophoresis techniques. Molecules 24(24):4617. https://doi.org/10.3390/molecules24244617
Deeb SE, Watzig H, El-Hady DA, Albishri HM, Sanger-van de Griend C, Scriba GKE (2014) Recent advances in capillary electrophoretic migration techniques for pharmaceutical analysis. Electrophoresis 35:170–189
Suntornsuk L, Anurukvorakun O (2022) Sensitivity enhancement in capillary electrophoresis and their applications for analyses of pharmaceutical and related biochemical substances. Electrophoresis 43(9–10):939–954
Bavetsias V, Lanigan RM, Ruda GF, Atrash B, McLaughlin MG, Tumber A, Blagg J (2016) 8-Substituted pyrido[3,4-d]pyrimidin-4(3H)-one derivatives as potent, cell permeable, KDM4 (JMJD2) and KDM5 (JARID1) histone lysine demethylase Inhibitors. J Med Chem 59(4):1388–1409. https://doi.org/10.1021/acs.jmedchem.5b01635
Bazgir A, Khanaposhtani MM, Soorki AA (2008) One-pot synthesis and antibacterial activities of pyrazolo[4′,3′:5,6]pyrido[2,3-d]pyrimidine-dione derivatives. Bioorg Med Chem Lett 18(21):5800–5803. https://doi.org/10.1016/j.bmcl.2008.09.057
El-Gazzar A-RBA, Hafez HN (2009) Synthesis of 4-substituted pyrido[2,3-d]pyrimidin-4(1H)-one as analgesic and anti-inflammatory agents. Bioorg Med Chem Lett 19(13):3392–3397. https://doi.org/10.1016/j.bmcl.2009.05.044
Kang MA, Kim M-S, Kim JY, Shin Y-J, Song Y-J, Jeong J-H (2014) A novel pyrido-thieno-pyrimidine derivative activates p53 through induction of phosphorylation and acetylation in colorectal cancer cells. Int J Oncol 46(1):342–350. https://doi.org/10.3892/ijo.2014.2720
Lakshmi Narayana B, Ram Rao AR, Shanthan Rao P (2009) Synthesis of new 2-substituted pyrido[2,3-d]pyrimidin-4(1H)-ones and their antibacterial activity☆. Eur J Med Chem 44(3):1369–1376. https://doi.org/10.1016/j.ejmech.2008.05.025
La Motta C, Sartini S, Mugnaini L, Simorini F, Taliani S, Salerno S, Ciuffi M (2007) Pyrido[1,2-a]pyrimidin-4-one derivatives as a novel class of selective aldose reductase inhibitors exhibiting antioxidant activity. J Med Chem 50(20):4917–4927. https://doi.org/10.1021/jm070398a
Pasquier B, El-Ahmad Y, Filoche-Rommé B, Dureuil C, Fassy F, Abecassis P-Y, Ronan B (2014) Discovery of (2S)-8-[(3R)-3-methylmorpholin-4-yl]-1-(3-methyl-2-oxobutyl)-2-(trifluoromethyl)-3,4-dihydro-2H-pyrimido[1,2-a]pyrimidin-6-one: a novel potent and selective inhibitor of Vps34 for the treatment of solid tumors. J Med Chem 58(1):376–400. https://doi.org/10.1021/jm5013352
Ren Q, Liang Y-J, He H, Fu L, Gu Y (2009) A facile synthesis and biological activity of novel tetrahydrobenzo[4′,5′]thieno[3′,2′:5,6]pyrido[4,3-d]pyrimidin-4(3H)-ones. Bioorg Med Chem Lett 19(23):6713–6716. https://doi.org/10.1016/j.bmcl.2009.09.117
Wang T, Duncan L, Gu W, O’Dowd H, Wei Y, Perola E, Charifson PS (2012) Design, synthesis and biological evaluation of potent NAD+-dependent DNA ligase inhibitors as potential antibacterial agents. Part 2: 4-amino-pyrido[2,3-d]pyrimidin-5(8H)-ones. Bioorg Med Chem Lett 22(11):3699–3703. https://doi.org/10.1016/j.bmcl.2012.04.038
The Food and Drug Administration (FDA or Agency) Bioanalytical Method Validation. May 2018, Docket Number: FDA-2013-D-1020
International Council on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use. ICH Topic Q 2 (R1). Validation of Analytical Procedures: Text and Methodology. Step 5. Accessed 17 Aug 2021
Acknowledgements
The analytical studies were performed in the Chair and Department of Biochemistry and Pharmacogenomics of Medical University of Warsaw and financially supported by statutory funds of the WUM in Poland.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
BG designed and performed all experiments. BG performed a statistical analysis of the results. BG wrote the paper. GS proofread the work. MK, GS, PS and FH provided compounds for testing.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no conflict of interest.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
The studies were not conducted on humans or living organisms.
Permission and/or Credit for Reproduced Images
All authors consent to reproduction of images.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Grodner, B., Król, M., Ślifirski, G. et al. Determination of New 4-Aryl-pyrido[1,2-c]pyrimidine Derivatives, Potential Antidepressant Agents with a High Affinity to 5-Hydroxytryptamin 1A Receptor and Serotonin Transporter Protein Receptor, with Capillary Electrophoresis. Chromatographia 87, 117–124 (2024). https://doi.org/10.1007/s10337-023-04300-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10337-023-04300-0