Abstract
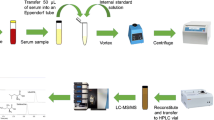
For the extraction and measurement of plasma amino acids using LC/ESI–MS/MS technology, a straightforward and dependable approach has been established. The approach employs a small amount of sample and fully quantifies it using a labeled internal standard of amino acids. Water, formic acid, and methanol were used as the mobile phase in a gradient method to obtain clear separation. On a Shimatzu mass spectrometer 8030, optimized multiple reaction monitoring (MRM) was utilized to find amino acids. All amino acids have coefficients of correlation that range from 0.91 to 0.99, and they are all linear over their respective reference ranges. Precision’s intra-day and inter-day coefficients of variation (CV %) ranged from 3.29 to 11.73% and 5.04 to 12.48%, respectively. Amino acid recovery ranged from 92.1 to 108.2%.

Similar content being viewed by others
Data availability
The data that support the findings of this study are available on request from the corresponding author (shrimant.p@gd-lab.com).
Abbreviations
- HPLC:
-
High-performance liquid chromatography
- GCMS:
-
Gas chromatography–mass spectrometry
- IEM:
-
Inborn error of metabolism
- TMS:
-
Tandem mass spectrometry
- ESI:
-
Electrospray ionization
- µL:
-
Micro liter
- µM·L-1 :
-
Micromole per liter
- MRM:
-
Multiple reaction monitoring
- RT:
-
Retention time
References
Michael A, Karen J, Nichole AR (2007) Analysis of 25 underivatized amino acids in human plasma using ion-pairing reversed-phase liquid chromatography/time-of-flight mass spectrometry. Rapid Commun Mass Spectrom 21:2717–2726
Suresh B, Shareef M, PavanKumar A, Taranath K (2002) HPLC method for amino acids profile in biological fluids and inborn metabolic disorders of aminoacidopathies. Indian J Clin Biochem 17(2):7–26
Elisabeth L, William L, Marzia P (2005) Analysis of plasma amino acids by HPLC with photodiode array and fluorescence detection. Clin Chim Acta 354:83–90
Robitaille L, Hoffer LJ (1988) Measurement of branched chain amino acids in blood plasma by high performance liquid chromatography. Can J Physiol Pharmacol 66:613–617
Schadewaldt P, Bodner-Leidecker A, Hammen HW, Wendel U (1999) Significance of l-alloisoleucine in plasma for diagnosis of maple syrup urine disease. Clin Chem 45:1734–1740
Schadewaldt P, Bodner-Leidecker A, Hammen HW, Wendel U (2000) Formation of l-alloisoleucine in vivo: an l-[13C] isoleucine study in man. Pediatr Res 47:271–277
Tom T, Paul L, Alexander H (1994) Plasma amino acids determined by liquid chromatography within 17 minutes. Clin Chem 40(2):245–249
Monique P, Christine V, Konstantinos P, Claire E, Jean-Paul S, Denis B (2005) Ion-pairing reversed-phase liquid chromatography/electrospray ionization mass spectrometric analysis of 76 underivatized amino acids of biological interest: a new tool for the diagnosis of inherited disorders of amino acid metabolism. Rapid Commun Mass Spectrom 19:1587–1602
Michael P, Robert W (2007) Simple and rapid quantitative high-performance liquid chromatographic analysis of plasma amino acids. J Chromatogr B Analyt Technol Biomed Life Sci 852(1–2):646–649
Shrimant P, Mahesh H, Susheel S, Akshata S, Pallavi K (2019) Fast & reliable liquid chromatography mass spectrometry method for determination of amino acids & acylcarnitines including succinyl acetone in dried blood spots for newborn screening. Int J Med Sci 6(7):1–11
Mahesh H, Pramod I, Shrimant P, Ashwini Y (2015) Two tier analysis of organic acid disorders: a comprehensive approach for newborn screening. Int J of Biomed Adv Res 6(02):84–90
Prinsen HCMT, Schiebergen-Bronkhorst BGM, Roeleveld MW, Jans JJM, Sain-van M, Visser G, van Hasselt PM, Verhoeven-Duif NM (2016) Rapid quantification of underivatised amino acids in plasma by hydrophilic interaction liquid chromatography (HILIC) coupled with tandem mass-spectrometry. J Inherit Metab Dis 39:651–660
Hariharan M, Sundar N, Ted V (1993) Systematic approach to the development of plasma amino acid analysis by high-performance liquid chromatography with ultraviolet detection with precolumn derivatization using phenyl isothiocyanate. J Chromatogr 621:15–22
Huffman K, Shah S, Stevens R, Bain JR, Muehlbauer M (2009) Relationships between circulating metabolic intermediates and insulin action in overweight to obese, inactive men and women. Diabetes Care 32:1678–1683
Newgard CB, An J, Bain JR, Muehlbauer MJ, Stevens RD et al (2009) A branched-chain amino acid-related metabolic signature that differentiates obese and lean humans and contributes to insulin resistance. Cell Metab 9:311–326
Tai ES, Tan ML, Stevens RD, Low YL, Muehlbauer MJ et al (2010) Insulin resistance is associated with a metabolic profile of altered protein metabolism in Chinese and Asian-Indian men. Diabetologia 53:757–767
Wang TJ, Larson MG, Vasan RS, Cheng S, Rhee EP (2011) Metabolite profiles and the risk of developing diabetes. Nat Med 17:448–453
Wurtz P, Soininen P, Kangas AJ, Ronnemaa T, Lehtimaki T (2013) Branched-chain and aromatic amino acids are predictors of insulin resistance in young adults. Diabetes Care 36:648–655
Laferrere B, Reilly D, Arias S, Swerdlow N, Gorroochurn P (2011) Differential metabolic impact of gastric bypass surgery versus dietary intervention in obese diabetic subjects despite identical weight loss. Sci Transl Med 3:80–82
Hsiao G, Chapman J, Ofrecio JM, Wilkes J, Resnik JL (2011) Multi-tissue, selective PPARgamma modulation of insulin sensitivity and metabolic pathways in obese rats. Am J Physiol Endocrinol Metab 300:E164-174
Shah SH, Crosslin DR, Haynes CS, Nelson S, Turer CB (2012) Branched chain amino acid levels are associated with improvement in insulin resistance with weight loss. Diabetologia 55:321–330
Piraud M, Vianey-Saban C, Petritis K, Elfakir C, Steghens JP, Bouchu D (2005) Ion-pairing reversed-phase liquid chromatography/electrospray ionization mass spectrometric analysis of 76 underivatized amino acids of biological interest: a new tool for the diagnosis of inherited disorders of amino acid metabolism. Rapid Commun Mass Spectrom 19:1587–1602
Waterval WA, Scheijen JL, Ortmans-Ploemen MM, Habets-van der Poel CD, Bierau J (2009) Quantitative UPLC-MS/MS analysis of underivatised amino acids in body fluids is a reliable tool for the diagnosis and follow-up of patients with inborn errors of metabolism. Clin Chim Acta 407:36–42
Acknowledgements
We are grateful to Mr. Abhimanyu Kumar, CEO and Mr. Susheel Sing CTO of General Diagnostics International Pvt Ltd, Mumbai (India), for their institutional funding, continuing interest, encouragement, and administrative support.
Funding
The authors declare that they have no any external agency funding for the present work.
Author information
Authors and Affiliations
Contributions
All authors equally contributed to the study conception and design, material preparation, data collection, and analysis.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Panaskar, S.N., Singh, S.K. Quantification of Amino Acids in Plasma by High-Performance Liquid Chromatography–Tandem Mass Spectrometry (LC–MS/MS). Chromatographia 86, 567–572 (2023). https://doi.org/10.1007/s10337-023-04262-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10337-023-04262-3