Abstract
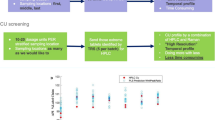
As a result of the excipient materials added to the drug substance to create a formulated pharmaceutical product, the preparation of drug product samples for chromatographic or other quantitative analysis can be manually intensive and time-consuming. It is therefore important that enough focus is placed on this aspect of method development to ensure accurate and reproducible results are obtainable across different laboratories. This paper outlines some of the challenges encountered when developing quantitative assay methods which are attributable to sample preparation procedures for solid-oral dosage forms (i.e. tablets and capsules). Approaches utilising experimental design, data visualisation and process understanding tools are described to highlight the importance of common sample preparation factors on drug recovery in product formulations. For a tablet formulation, experimental design highlighted that the percentage of organic in the sample diluent and the orbital shaker speed were statistically significant and could impact accuracy of assay result. For the second formulation, a gelatin capsule, the use of experimental design and the Britest process understanding tools highlighted the initial volumetric flask fill volume required close control to ensure reproducible assay results while the stand time (time sample left to stand post-shaking to aid analyte solubilisation) and capsule storage conditions were also significant.









Similar content being viewed by others
References
Nickerson B (2011) Sample preparation of pharmaceutical dosage forms. Springer, New York
Imamoglu E, Tunca AK (2014) Simultaneous determination of guaifenesin and ephedrine HCl binary mixtures in syrup dosage forms and human plasma by RP-HPLC-DAD. J Liq Chromatogr Rel Technol 37:1039–1051
Harrington B, Nickerson B, Guo MX, Barber M, Giamalva D, Lee C, Scrivens G (2014) Sample preparation composite and replicate strategy for assay of solid oral drug products. Anal Chem 86:11930–11936
Kataoka H, Lord HL (2002) In: Pawliszyn J (ed) Comprehensive Analytical Chemistry XXXVII. Elsevier, Amsterdam
Moldoveanu SC (2004) Solutions and challenges in sample preparation for chromatography. J Chromatogr Sci 42:1–14
Separovic L, Lourenco FR, Measurement (2019) Uncertainty and risk of false conformity decision in the performance evaluation of liquid chromatography analytical procedures. J Pharm Biomed Anal 171:73–80
Hotha KK, Roychowdhury S, Subramanian V (2016) Drug-excipient interactions: case studies and overview of drug degradation pathways. Am J Anal Chem 7:107–140
Larsen J, Melander C (2012) Study of interaction between crosscaramellose and escitalopram during sample preparation. Drug Dev Ind Pharm 38:1195–1199
Wu Y, Levons J, Narang AS, Raghavan K, Rao VM (2011) Reactive impurities in excipients: profiling, identification and mitigation of drug-excipient incompatibility. AAPS PharmSci Tech 12:1248–1263
Celestino MT, de Oliveira Magalhães U, Fraga AGM, Almada do Carmo F, Lione V, Castro HC, Sousa VP, Rodrigues CR, Cabral LM (2012) Rational use of antioxidants in solid oral pharmaceutical preparations BJPS 48:405–415
Wasylaschuk WR, Harmon PA, Wagner G, Harma AB, Templeton AC, Xu H, Reed RA (2007) Evaluation of hydroperoxides in common pharmaceutical excipients. J Pharm Sci 96:106–116
Skibic MJ, King LA, Khan M, Fox PJ, Winger BE, Baertschi SW (2010) Artifactual formylation of the secondary amine of duloxetine hydrochloride by acetonitrile in the presence of titanium dioxide: implications for method development. J Pharm Biomed Anal 53:432–439
Nickerson B, Arikpo WB, Berry MR, Bobin VJ, Houck TL, Mansour HL, Warzeka J (2008) Leveraging elevated temperature and particle size reduction to extract API from various tablet formulations. J Pharm Biomed Anal 47:268–278
Kok SJ, Debets AJJ (2001) Fast sample preparation for analysis of tablets and capsules: the ball mill extraction method. J Pharm Biomed Anal 26:599–604
Lee C, Gallo J, Arikpo W, Bobin V (2007) Comparison of extraction techniques for spray dried dispersion tablet formulations. J Pharm Biomed Anal 45:565–571
Opio AM, Nickerson B, Xue G, Warzeka J, Norris K (2011) Expanding the application of tablet processing workstation to support the sample preparation of oral suspensions. J Assoc Lab Auto JALA 16:229–234
Cela R, Ordóñez EY, Quintana JB, Rodil R (2013) Chemometric-assisted methods development in reversed-phase liquid chromatography. J Chromatogr A 1287:2–22
Wang L, Zheng J, Gong X, Hartman R, Antonucci V (2015) Efficient HPLC method development using structure-based database search, physico-chemical prediction and chromatographic simulation. J Pharm Biomed Anal 104:49–54
Zöldhegyi A, Rieger H-J, Molnár I, Fekhretdinova L (2018) Automated UHPLC separation of 10 pharmaceutical compounds using software-modeling. J Pharm Biomed Anal 156:379–388
Harang V, Karlsson A, Josefson M (2001) Liquid chromatography method development and optimization by statistical experimental design and chromatogram simulations. Chromatographia 54:703–709
Jovanović M, Rakić T, Tumpa A, Stojanović B-J (2015) Quality by Design approach in the development of hydrophilic interaction liquid chromatographic method for the analysis of iohexol and its impurities. J Pharm Biomed Anal 110:42–48
Box GEP, Hunter JS, Hunter WG (1978) Stat Exp. Wiley, New York
Springall J (2018) How to Utilize Design of Experiment (DoE) Principles for Developing Robust Analytical Methods for QC Environments Am. Pharm. Rev. https://www.americanpharmaceuticalreview.com/Featured-Articles/348755-How-to-Utilize-Design-of-Experiment-DoE-Principles-for-Developing-Robust-Analytical-Methods-for-QC-Environments/. Accessed 11 Feb 2020
Dejaegher B, Heyden Y (2009) Response surface designs (part 1) types and properties. LCGC Europe 22:256–261
Dejaegher B, Van der Heyden Y (2009) Response surface designs (part 2)—data analyis and multiresponse optimization. LCGC Europe 22:581–585
Xing L, Glen RC (2002) Novel methods for the prediction of logP, pKa and logD. J Chem Inf Comput Sci 42:796–805
Wrezel PW, Chion I, Pakula R (2005) Key factors in sample diluent selection for HPLC assays of active pharmaceutical ingredients. LCGC North Am 29:107–111
Kazakevich Y, LoBrutto R (2007) HPLC for Pharmaceutical Scientists. Wiley, New York
Iwasaki T, Maegawa Y, Ohshima T, Mashima K (2012) Esterification https://onlinelibrary.wiley.com/doi/10.1002/0471238961.0519200501191201.a01.pub2. Accessed 11 Feb 2020
Kepner CH, Tregoe BB (2008) The New Rational Manager. Princeton Research Press, New Jersey
Meyer VR (2003) Measurement uncertainty of liquid chromatographic analyses visualized by ishikawa diagrams. J Chromatogr Sci 41:439–443
Kenett RS (2008) Cause and effect diagrams in Encyclopedia of statistics in quality and reliability https://doi.org/10.1002/9780470061572.eqr413. Accessed 11 Feb 2020
Shaw N, Burgess T, Hwarng H, de Mattos C (2001) Revitalising new process development in the UK fine chemicals industry. Int J Operat Prod Manage 21(8):1133–1151
Britest Ltd (1999) Daresbury, UK. https://www.britest.co.uk. Accessed 12 Nov 2019
Britest Ltd (1999) Case studies Daresbury, UK: https://www.britest.co.uk/case_studies/. Accessed 12 Nov 2019
Lachlan KM, Gordon C, Glassey J (2017) Qualitative process understanding tools within bioprocessing: a case study. J Bioprocess Biotech 7:305. https://doi.org/10.4172/2155-9821.1000305
Dejaegher B, Jimidar M, De Smet M, Cockaerts P, Smeyers-Verbeke J, Vander Heyden Y (2006) Improving method capability of a drug substance HPLC assay. J Pharm Biomed Anal 42:155–170
Chatfield MJ, Borman PJ (2009) Acceptance criteria for method equivalency assessments. Anal Chem 81:9841–9848
Separovic L, Morais A, Felipe S, Lourenço R (2018) Using measurement uncertainty to assess the fitness for purpose of an HPLC analytical method in the pharmaceutical industry. Measurement 119:41–45
Erner J (2018) Analytical Target Profile: establishment of precision requirements for assay. J Pharm Biomed Anal 160:73–79
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ferguson, P.D., Shaw, R., McCudden, A. et al. Improving Robustness of Pharmaceutical Dosage form Sample Preparation Using Experimental Design and Process Understanding Tools. Chromatographia 83, 1525–1538 (2020). https://doi.org/10.1007/s10337-020-03969-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10337-020-03969-x