Abstract
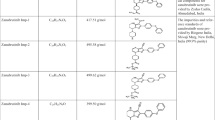
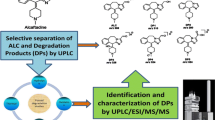
Enzalutamide was recently approved for the treatment of castration-resistant prostate cancer. In this study, the related substances in enzalutamide bulk substance were analyzed qualitatively and quantitatively. Four degradation products (Oxi, A9, P1 and P2) of enzalutamide were isolated using semi-preparative liquid chromatography and characterized using nuclear magnetic resonance and mass spectrometry. In addition, two reversed-phase liquid chromatography methods were developed for quantification of 13 potential impurities and the main constituent, respectively. These two methods were validated and applied during the impurity studies and quality control analysis of the laboratory-prepared samples of enzalutamide.











Similar content being viewed by others
References
Sanford M (2013) Enzalutamide, a review of its use in chemotherapy-naive metastatic castration-resistant prostate cancer. Drugs 73:1723–1732
Cella D, Ivanescu C, Holmstrom S, Bui CN, Spalding J, Fizazi K (2015) Impact of enzalutamide on quality of life in men with metastatic castration-resistant prostate cancer after chemotherapy: additional analyses from the AFFIRM randomized clinical trial. Ann Oncol 26:179–185
(2018) Full prescribing information for XTANDI, Astellas Pharma US, Inc.
Axel Heidenreich S, Chowdhury L, Klotz DR, Siemens et al (2017) Impact of enzalutamide compared with bicalutamide on quality of life in men with metastatic castration-resistant prostate cancer: additional analyses from the TERRAIN randomised clinical trial. Eur Urol 71:534–542
Beer TM, Armstrong AJ, Rathkopf DE et al (2014) Enzalutamide in metastatic prostate cancer before chemotherapy. N Engl J Med 371:424–433
Puszkiel A, Plé A, Huillard O, Noé G, Thibault C, Oudard S, Goldwasser F, Vidal M, Alexandre J, Blanchet B (2017) A simple HPLC-UV method for quantification of enzalutamide and its active metabolite N-desmethyl enzalutamide in patients with metastatic castration-resistant prostate cancer. J Chromatography B. https://doi.org/10.1016/j.jchromb.2017.04.014
Kim KP, Parise RA, Holleran JL, Lewis LD, Appleman L, van Erp N, Morris MJ, Beumer JH (2017) Simultaneous quantitation of abiraterone, enzalutamide, N-desmethyl enzalutamide, and bicalutamide in human plasma by LC–MS/MS. J Pharm Biomed Anal. https://doi.org/10.1016/j.jpba.2017.02.018
Ma X, Zhou W, Zou Q, Ouyang P (2016) Structural elucidation of the impurities in Enzalutamide bulk drug and the development, validation of corresponding HPLC method. J Pharm Biomed Anal. https://doi.org/10.1016/j.jpba.2016.08.036
(2006) ICH harmonized tripartite guideline, impurities in new drug substances Q3A (R2), current step 4 version, 25 October 2006
ICH Topic Q1A (R2) (2003) Stability testing of new drug substances and products. In: Proceedings of the international conference on harmonization. EMEA, London
Acknowledgements
The work was supported by the 2016 Shanghai Pujiang Talent Plan (No. 16PJ1432800).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Rights and permissions
About this article
Cite this article
Zhou, A., Ruan, L., Duan, G. et al. Quantitative Study of Impurities in Enzalutamide and Identification, Isolation, Characterization of Its Four Degradation Products Using HPLC, Semi-preparative LC, LC/ESI–MS and NMR Analyses. Chromatographia 81, 1519–1531 (2018). https://doi.org/10.1007/s10337-018-3611-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10337-018-3611-4