Abstract
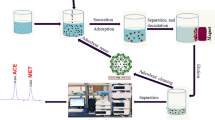
In this research, a new type of poly ionic liquid-coated magnetic nanoparticle (MNPs@PIL) was designed and prepared via free radical polymerization. The as-synthesized MNPs were characterized using Fourier transform infrared spectroscopy (FT-IR), X-ray diffraction (XRD), scanning electron microscopy (SEM), transmission electron microscopy (TEM), thermo gravimetric analysis (TGA) and elemental analysis (EA). Then, they were successfully used as adsorbent in magnetic solid-phase extraction (MSPE) method for the extraction of nitrate and nitrite anions from water samples, and the analytes were finally determined by ion chromatography/conductivity detection. The effects of several experimental variables (including pH of sample solution, amount of the adsorbent, extraction and desorption time) were studied and the optimum values were determined. Under the optimum experimental conditions, detection limits (S/N = 3) of the proposed method for nitrate and nitrite were obtained as 0.2 and 0.133 µg L−1, respectively. The repeatability of the method, expressed as relative standard deviation (RSD), was lower than 5%. The proposed method was successfully applied for determination of nitrate and nitrite in real water samples.
Graphical abstract











Similar content being viewed by others

References
Aguilar-Arteaga K, Rodriguez JA, Barrado E (2010) Anal Chim Acta 674:157–165
Feizbakhsh A, Ehteshami S (2016) Chromatographia 79:1177–1185
Vasconcelos I, Fernandes Ch (2017) TrAC Trends Anal Chem 89:41–52
Wu L, Yu L, Ding X, Li P, Dai X, Chen X, Zhou H, Bai Y, Ding J (2017) Food Chem 217:320–325
Qin X, Ahmed Bakheet AA, Zhu X (2017) J Iran Chem Soc 14:2017–2022
Nasiri J, Naghavi MR, Alizadeh H, Fattahi-Moghadam MR, Motamedi E, Mashouf A (2015) Chromatographia 78:1143–1157
Nazari Serenjeh F, Hashemi P, Naeimi H, Zakerzadeh E, Ghiasvand AR (2016) Microchim Acta 183(8):2449–2455
Keskin S, Kayrak-Talay D, Akman U, Hortacsu O (2007) J Supercrit Fluids 43:150–180
Yuan JY, Antonietti M (2011) Polymer 52(7): 1469–1482
Yuan JY, Mecerreyes D, Antonietti M (2013) Prog Polym Sci 38:1009–1036
Devasurendra AM, Zhang C, Young JA, Tillekeratne LMV, Anderson JL, Kirchhoff JR (2017) ACS Appl Mater Interfaces 9(29):24955–24963
Young JA, Zhang C, Devasurendra AM, Tillekeratne LMV, Anderson JL, Kirchhoff JR (2016) Anal Chim Acta 910:45–52
Huang XJ, Chen LL, Yuan DX, Bi SS (2012) J Chromatogr A 1248:67–73
Galan-Cano F,. Alcudia-Leon MDC, Lucena R, Cardenas S, Valcarcel M (2013) J Chromatogr A 1300:134–140
Bi W, Wang M, Yang X, Ho-Row K (2014) J Sep Sci 37:1632–1639
Yang L, Su P, Chen X, Zhang R, Yang Y (2015) Anal Methods 7:3246–3252
Huang X, Wang Y, Liu Y, Yuan D (2013) J Sep Sci 36:3210–3219
Ahmed-Bakheet AA, Shi-Zhu X (2017) J Mol Liq 242:900–906
Moorcroft MJ, Davis J, Compton RG (2001) Talanta 54:785–803
Wang QH, Yu LJ, Liu Y, Lin L, Lu R, Zhu J, He L, Lu ZL (2017) Talanta 165:709–720
Antczak-Chrobot A, Bak P, Wojtczak M (2018) Food Chem 240:648–654
Kissner R, Koppenol WH (2005) Methods Enzymol 396:61–68
Helaleh MIH, Korenaga T (2000) J Chromatogr B 744:433–437
Muscara MN, De Nucci G (1996) J Chromatogr B 686:157–164
Mashhadizadeh MH, Karami Z (2011) J Hazard Mater 190:1023–1029
Wu JW, Su P, Huang J, Wang SM, Yang Y (2013) J Colloid Interface Sci 399:107–109
Deng YH, Qi D, Deng CH, Zhang XM, Zhao DY (2008) J Am Chem Soc 130:28–31
Kaykhaii M, Dicinoski GW, Smedley R, Pawliszyn J, Haddad PR (2010) J Chromatogr A 1217:3452–3456
Hu ZZ, Chen HD, Yao CY, Zhu Y (2011) J Chromatogr Sci 49:612–617
Acknowledgements
The authors wish to thank Imam Khomeini international university research council for supporting the project.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors acknowledge that there was no conflict of interest.
Ethical Approval
There were no human or animals test participants involved in this study.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Aflatouni, F., Soleimani, M. Preparation of a New Polymerized Ionic Liquid-Modified Magnetic Nano Adsorbent for the Extraction and Preconcentration of Nitrate and Nitrite Anions from Environmental Water Samples. Chromatographia 81, 1475–1486 (2018). https://doi.org/10.1007/s10337-018-3590-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10337-018-3590-5