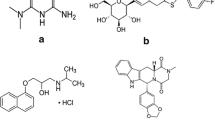
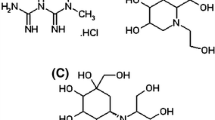
Abstract
A new liquid chromatography tandem mass spectrometric method for simultaneous estimation of anti-hyperglycemic agents, metformin, linagliptin, sitagliptin and vildagliptin from human plasma was developed and validated as per ICH/USFDA guidelines. Chromatographic separations were achieved using Chromolith® High Resolution RP-18e HPLC column (100 mm × 4.6 mm, macropores 1.15 μm) with an isocratic elution mode using the mobile phase composed of 0.01 M ammonium formate buffer (pH 3.0): acetonitrile (80:20 v/v). A flow rate of 0.4 mL min−1 was maintained throughout the analysis. Detection was performed by triple quadrupole MS fitted with ESI probe functioning in the positive ion MRM mode. Extractions of all the drugs from plasma were carried out by acetonitrile crash technique. Alogliptin was used as an internal standard to minimize the error which could occur during analysis. The validation study data demonstrated that the new method is highly selective and sensitive (the limits of detection were 1.76, 1.94, 0.17 and 3.08 ng mL−1 for metformin, linagliptin, sitagliptin and vildagliptin, respectively). The %CV and %RE values were within the acceptable limit, <1% for most of the data, which indicate that the reported method was very precise and accurate. A linear calibration curve (correlation coefficient, r 2 > 0.999) was obtained at the concentration range of 0.5–400.0; 5.0–400.0; 10.0–500.0 and 0.5–40.0 ng mL−1 for metformin, linagliptin, sitagliptin and vildagliptin, respectively. The extraction efficacy was evidenced from recovery study and all four analytes were found to be stable in plasma. The developed LC–MS/MS method is robust and can be applied for monitoring plasma levels of analyzed antidiabetic drugs in preclinical and clinical pharmacokinetic studies.
Graphical abstract



Similar content being viewed by others
References
Fisman EZ, Tenenbaum A (2015) Antidiabetic treatment with gliptins: focus on cardiovascular effects and outcomes. Cardiovasc Diabetol 14:129–141
Chahal H, Chowdhury TA (2007) Gliptins: a new class of oral hypoglycaemic agent. Q J Med 100:671–677
Moses R (2009) Fixed combination of repaglinide and metformin in the management of type 2 diabetes. Diabetes Metab Syndr Obes 2:101–109
Reddy S, Ahmed I, Ahmad I, Mukhopadhyay A, Thangam S (2015) Development and validation of a method for simultaneous estimation of metformin and sitagliptin in human plasma by LC–MS-MS and its application in a bioequivalence study. J Chromatogr Sci 53:1549–1556
Herman GA, Bergman A, Liu F, Stevens C, Wang AQ, Zeng W, Chen L, Snyder K, Hilliard D, Tanen M, Tanaka W, Meehan AG, Lasseter K, Dilzer S, Blum R, Wagner JA (2006) Pharmacokinetics and pharmacodynamic effects of the oral DPP-4 inhibitor sitagliptin in middle-aged obese subjects. J Clin Pharmacol 46:876–886
Gupta V, Kalra S (2011) Choosing a gliptin. Indian J Endocrinol Metabol 15:298–308
Esposito K, Cozzolino D, Bellastella G, Maiorino MI, Chiodini P, Ceriello A, Giugliano D (2011) Dipeptidyl peptidase-4 inhibitors and HbA1c target of <7% in type 2 diabetes: meta-analysis of randomized controlled trials. Diabetes Obes Metabol 13:594–603
Bodar JD, Kumar S, Yadav YC, Seth AK, Deshmukh GJ, Sen AK, Shah A (2011) Development of the spectrophotometric method for the simultaneous estimation of pioglitazone and metformin. Pharm Sci Monit 2:236–243
Doredla NR, Shanmugasundaram P, Vaishnav H (2011) Method development and validated of simultaneous estimation of metformin hydrochloride, pioglitazone hydrochloride and glibenclamide in pure and tablet dosage form by spectrophotometric multi component method. Int J Chem Tech Res 3:2011–2017
Lakshmi KS, Rajesh T, Sharma S (2009) Simultaneous determination of metformin and pioglitazone by Reversed phase HPLC in pharmaceutical dosage forms. Int J Pharm Pharm Sci 1:162–166
Chhetri HP, Thapa P, Achepdael AV (2014) Simple HPLC-UV method for the quantification of metformin in human plasma with one step protein precipitation. Saudi Pharm J 22:483–487
Mansoory NM, Jain A (2012) Simultaneous estimation of metformin hydrochloride, pioglitazone hydrochloride and gliclazide by validated RP-HPLC method in solid dosage form. Int J Pharm Pharm Sci 4:72–76
Gite S, Patravale V (2015) Validation of RP-HPLC method and stress degradation for the combination of metformin HCl, atorvastatin calcium and glimepiride: application to nanoparticles. J Chromatogr Sci 53:1654–1662
Koseki N, Kawashita H, Nina M, Nagae Y, Masuda N (2005) Development and validation for high selective quantitative determination of metformin in human plasma by cation exchanging with normal-phase LC/MS/MS. J Pharm Biomed Anal 36:1063–1072
Sharma K, Pawar G, Yadam S, Giri S, Rajagopal S, Mullangi R (2013) LC–MS/MS-ESI method for simultaneous quantitation of metformin and repaglinidie in rat plasma and its application to pharmacokinetic study in rats. Biomed Chromatogr 27:356–364
Wang Y, Tang Y, Gu J, Fawcett JP, Bai X (2004) Rapid and sensitive liquid chromatography-tandem mass spectrometric method for the quantitation of metformin in human plasma. J Chromatogr B 808:215–219
Chen X, Gu Q, Qiu F, Hong D (2004) Rapid determination of metformin in human plasma by liquid chromatography-tandem mass spectrometry method. J Chromatogr B 802:377–381
Hamdana II, Bani Jaber AK, Abushoffa AM (2010) Development and validation of a stability indicating capillary electrophoresis method for the determination of metformin hydrochloride in tablets. J Pharm Biomed Anal 53:1254–1257
Doomkaew A, Prutthiwanasan B, Suntornsuk L (2014) Simultaneous analysis of metformin and cyanoguanidine by capillary zone electrophoresis and its application in a stability study. J Sep Sci 37:1687–1693
Khan G, Sahu D, Agrawal YP, Sabarwal N, Jain A, Gupta AK (2011) Simultaneous estimation of metformin and sitagliptin in tablet dosage form. Asian J Biochem Pharm Res 1:352–358
El-Bagary RI, Elkady EF, Ayoub BM (2011) Spectroflourometric and spectrophotometric methods for the determination of sitagliptin in binary mixture with metformin and ternary mixture with metformin and sitagliptin alkaline degradation product. Int J Biomed Sc 7:62–69
Vishal G, Rajeshwari R, Ranjeet D, Manish N (2013) Simultaneous quantification of aliskire, valsartan and sitagliptin by LC with fluorescence detection: evidence of pharmacokinetic interaction in rats. Chromatographia 76:515–521
Salim M, El-Enany N, Belal F, Walash M, Patonay G (2012) Simultaneous determination of sitagliptin and metformin in pharmaceutical preparations by capillary zone electrophoresis and its application to human plasma analysis. Anal Chem Insights 7:31–46
El-Bagary RI, Elkady EF, Ayoub BM (2011) Liquid chromatographic determination of sitagliptin either alone or in ternary mixture with metformin and sitagliptin degradation product. Talanta 85:673–680
Suresh PS, Srinivas NR, Mullangi R (2016) A concise review of the bioanalytical methods for the quantitation of sitagliptin, an important dipeptidyl peptidase-4 (DPP4) inhibitor, utilized for the characterization of the drug. Biomed Chromatogr 30:749–771
Rezk MR, Riad SM, Mahmoud GY, Abdel Aleem AA (2013) Simultaneous determination of sitagliptin and metformin in their pharmaceutical formulation. J AOAC Int 96:301–306
Chellu SN, Malleswararao MV, Suryanarayana MK (2012) Simultaneous determination of sitagliptin phosphate monohydrate and metformin hydrochloride in tablets by a validated UPLC method. Sci Pharm 80:139–152
Zeng W, Xu Y, Constanzer M, Woolf EJ (2010) Determination of sitagliptin in human plasma using protein precipitation and tandem mass spectrometry. J Chromatogr B 878:1817–1823
Burugula L, Mullangi R, Pilli NR, Makula A, Lodagala DS, Kandhagatla R (2013) Simultaneous determination of sitagliptin and simvastatin in human plasma by LC–MS/MS and its application to a human pharmacokinetic study. Biomed Chromatogr 27:80–87
Hess C, Musshoff F, Madea B (2011) Simultaneous identification and validated quantification of 11 oral hypoglycaemic drugs in plasma by electrospray ionisation liquid chromatography–mass spectrometry. Anal Bioanal Chem 400:33–41
Martín J, Buchberger W, Santos JL, Alonso E, Aparicio I (2012) High performance liquid chromatography quadrupole time-of-flight mass spectrometry method for the analysis of antidiabetic drugs in aqueous environmental samples. J Chromatogr B 895:94–101
Vemula P, Dodda D, Balekari U, Panga S, Veeresham C (2015) Simultaneous determination of linagliptin and metformin by reverse phase-high performance liquid chromatography method: an application in quantitative analysis of pharmaceutical dosage forms. J Adv Pharm Technol Res 6:25–28
Shafi MSS, Begum A, Saradhi NDVR (2014) Bioanalytical method development and validation of linagliptin in plasma through LC–MS/MS. Int J Bioassays 3:3146–3152
Friedrich C, Shi X, Zeng P, Ring A, Woerle HJ, Patel S (2012) Pharmacokinetics of single and multiple oral doses of 5 mg linagliptin in healthy Chinese volunteers. Int J Clin Pharmacol Therap 50:889–895
Ashutosh KS, Manidipa D, Seshagiri RJVLN, Gowri SD (2015) New validated stability indicating RP-HPLC method for simultaneous estimation of metformin and alogliptin in human plasma. J Chromatogr Sep Tech 6:293
Sri GS, Kumar SA, Saravanan J, Debnath M, Greeshma V, Krishna NS (2013) A new stability indicating RP-HPLC method development for simultaneous estimation of metformin and alogliptin in bulk as well as in pharmaceutical formulation by using PDA detector. Indo Am J PR 3:9222–9241
de Andrade C, de Araujo Lock G, Piqatto MC, Haas SE, Costa TD, de Araujo BV (2014) Validation of LC–MS/MS method applied to evaluation of free tissue concentrations of vildagliptin in diabetic rats by microdialysis. Biomed Chromatogr 28:1722–1727
Barden AT, Salamon B, Schapoval EES, Steppe M (2012) Stability Indicating RP-LC method for the determination of vildagliptin and mass spectrometry detection for a main degradation product. J Chromatogr Sci 50:426–432
Pharne AB, Santhakumari B, Ghemud AS, Jain HK, Kulkarni MJ (2012) Bioanalytical method development and validation of vildagliptin a novel dipeptidyl peptidase IV inhibitor by RP-HPLC method. Int J Pharm Pharm Sci 4:119–123
Pednekar S, Lokhande R, Sutar R, Kolhal S, Surve S, Gudekar S (2014) Simultaneous determination of metformin, sitagliptin, saxagliptin, linagliptin and vildagliptin in multicomponent pharmaceutical preparations by RP-HPLC. Int J Pharm Sci Rev Res 28:128–133
Attimarad M, Nagaraja SH, Aldhubaib BE, Nair A, Venugopala KN (2014) Simultaneous determination of metformin and three gliptins in pharmaceutical formulations using RP HPLC: application to stability studies on linagliptin tablet formulation. Indian J Pharm Edu Res 48:45–53
International Conference on Harmonization (2000) Guidance for industry, validation of analytical procedures: methodology. Q2B Geneva, Switzerland
International Conference on Harmonization (2005) Validation of analytical procedures—text and methodology. Q2 R1 Geneva, Switzerland
Food and Drug Administration (2000) Analytical procedures and methods validation in chemistry, manufacturing and controls documentation. US Department of Health and Human Services, Maryland
Charde MS, Welankiwar AS, Kumar Jitendra, Chakole RD (2013) Bioanalytical method development and validation. Int J Adv Pharm Anal 3:90–94
Acknowledgements
The authors gratefully acknowledge Baish General Hospital, Saudi Arabia for providing human blood plasma for the study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Al Bratty, M., Alhazmi, H.A., Javed, S.A. et al. Development and Validation of LC–MS/MS Method for Simultaneous Determination of Metformin and Four Gliptins in Human Plasma. Chromatographia 80, 891–899 (2017). https://doi.org/10.1007/s10337-017-3288-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10337-017-3288-0