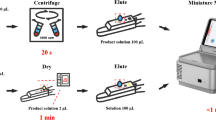
Abstract
This is a case study of the difficulties caused by supersaturation of an analyte during the quantification of a drug in urine. To support the clinical development of the potent helicase–primase inhibitor ASP2151, we developed and validated simple and semi-automated methods for determining its concentrations in human plasma and urine. Samples were mixed with an internal standard labeled with stable isotopes, and then filtered. After filtration, the samples were injected into an online extraction, column-switching, liquid chromatography–tandem mass spectrometry analytical system using a validated method. Although quantifiable and reproducible plasma concentrations were obtained with clinical samples, we encountered difficulties with urine samples. Specifically, we found that urinary drug concentrations in some samples ranged between 5 and 67.1 μg mL−1, and exceeded the aqueous saturation concentration (5 μg mL−1), at which dilution integrity was confirmed. In a follow-up experiment using spiked samples, the urine method failed to accurately quantitate ASP2151 concentrations at 10, 25, and 50 µg mL−1 (relative error: −23.5% to −17.2%, coefficient of variation: ≤7.9%). In vitro experiments revealed that urine samples at high ASP2151 concentrations became heterogeneous during sample handling and storage. The problem was solved by revising the sample collection and dilution methods which were subsequently successfully applied to a clinical study.
Graphical Abstract







Similar content being viewed by others
References
Chono K, Katsumata K, Kontani T et al (2010) ASP2151, a novel helicase-primase inhibitor, possesses antiviral activity against varicella-zoster virus and herpes simplex virus types 1 and 2. J Antimicrob Chemother 65:1733–1741. doi:10.1093/jac/dkq198
Katsumata K, Chono K, Sudo K et al (2011) Effect of ASP2151, a herpesvirus helicase-primase inhibitor, in a guinea pig model of genital herpes. Molecules 16:7210–7223. doi:10.3390/molecules16097210
Tyring S, Wald A, Zadeikis N et al (2012) ASP2151 for the treatment of genital herpes: a randomized, double-blind, placebo- and valacyclovir-controlled, dose-finding study. J Infect Dis 205:1100–1110. doi:10.1093/infdis/jis019
Stevenson L, Garofolo F, DeSilva B et al (2013) 2013 white paper on recent issues in bioanalysis: “hybrid”–the best of LBA and LCMS. Bioanalysis 5:2903–2918. doi:10.4155/bio.13.238
Hilhorst M, van Amsterdam P, Heinig K et al (2015) Stabilization of clinical samples collected for quantitative bioanalysis—a reflection from the European Bioanalysis Forum. Bioanalysis 7:333–343. doi:10.4155/bio.14.290
Ji AJ, Jiang Z, Livson Y et al (2010) Challenges in urine bioanalytical assays: overcoming nonspecific binding. Bioanalysis 2:1573–1586. doi:10.4155/bio.10.114
Wang PG, Zhang J, Gage EM et al (2006) A high-throughput liquid chromatography/tandem mass spectrometry method for simultaneous quantification of a hydrophobic drug candidate and its hydrophilic metabolite in human urine with a fully automated liquid/liquid extraction. Rapid Commun Mass Spectrom 20:3456–3464. doi:10.1002/rcm.2733
Xu Y, Du L, Rose MJ et al (2005) Concerns in the development of an assay for determination of a highly conjugated adsorption-prone compound in human urine. J Chromatogr B 818:241–248. doi:10.1016/j.jchromb.2005.01.004
Schwartz M, Kline W, Matuszewski B (1997) Determination of a cyclic hexapeptide (L-743 872), a novel pneumocandin antifungal agent in human plasma and urine by high-performance liquid chromatography with fluorescence detection. Anal Chim Acta 352:299–307. doi:10.1016/S0003-2670(97)00263-8
Fisher AL, DePuy E, Shih T et al (2001) Liquid chromatographic-tandem mass spectrometric urine assay for a highly metabolized cyclic ureidobenzenesulfonamide: issues concerning assay specificity and quality control preparation. J Pharm Biomed Anal 26:739–752. doi:10.1016/S0731-7085(01)00438-1
Chen C, Bajpai L, Mollova N et al (2009) Sensitive and cost-effective LC–MS/MS method for quantitation of CVT-6883 in human urine using sodium dodecylbenzenesulfonate additive to eliminate adsorptive losses. J Chromatogr B 877:943–947. doi:10.1016/j.jchromb.2009.02.045
Li W, Luo S, Smith HT et al (2010) Quantitative determination of BAF312, a S1P-R modulator, in human urine by LC–MS/MS: prevention and recovery of lost analyte due to container surface adsorption. J Chromatogr B 878:583–589. doi:10.1016/j.jchromb.2009.12.031
James CA (2008) Sample Preparation. In: Venn RF (ed) Principles and Practice of Bioanalysis, 2nd edn. CRC Press, Boca Raton, pp 19–39
Ohtsu Y, Otsuka S, Nakamura T et al (2015) Regulated bioanalysis of conformers—a case study with ASP2151 in dog plasma and urine. J Chromatogr B 997:56–63. doi:10.1016/j.jchromb.2015.05.028
FDA (2001) Guidance for industry: bioanalytical method validation. Rockville, MD
Yoshida T, Kurimoto I, Umejima H et al (2013) Effects of dissolved state of aminoalkyl methacrylate copolymer E/HCl on solubility enhancement effect for poorly water-soluble drugs. Colloid Polym Sci 291:1191–1199. doi:10.1007/s00396-012-2848-y
Acknowledgements
We thank Astellas Pharma employees and staff of the clinical pharmacology units and bioanalytical laboratories for their contribution to the clinical studies and K. Kato, M. Katashima, A. Miyashita, C. van den Beld, and K. Tabata of Astellas Pharma for their helpful discussions.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Yoshiaki Ohtsu, Radboud van Trigt, Kaori Takama, Dorien Groenendaal, Akitsugu Takada, and Kiyoshi Noguchi are employees of Astellas Pharma. Takeshi Nakamura is an employee of Shin Nippon Biomedical Laboratories. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in, or a financial conflict with, the subject matter or materials discussed in the manuscript other than those disclosed.
All subjects were informed of the nature and purpose of the study and their written informed consent was obtained before they participated.
Rights and permissions
About this article
Cite this article
Ohtsu, Y., van Trigt, R., Takama, K. et al. Quantification of ASP2151 in Human Plasma and Urine: A Pitfall Associated with Supersaturation of Analyte in Urine. Chromatographia 80, 217–227 (2017). https://doi.org/10.1007/s10337-016-3236-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10337-016-3236-4