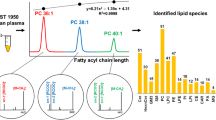
Abstract
The analysis of triacylglycerol (TG) species is of interest in diseases that are associated with hypertriglycidemia. The individual composition of TGs seems to be of special interest in the development of atherosclerosis or diabetes type II. Enzymatic methods based on saponification and glycerol analysis are not suited for the TG fatty acid distribution. The aim of the study was to develop a rapid method for a molecular species fingerprinting of TGs. Protein precipitation of 5 µL human plasma was carried out with toluene/methanol (1:1 v/v). Tandem mass spectrometric detection (mass range m/z 700–1,000) was performed by combination of nine neutral loss scans (14:0, 15:0, 16:0, 16:1, 18:0, 18:1, 18:2, 18:3, and 20:4) after flow-injection analysis for 2 min. Deuterated internal standards had been used for quantification. In human EDTA-plasma the detection limit was 3.3 µg/mL and the lower limit of quantification was 11.1 µg/mL. Linearity was proved for TG concentrations up to 100 µg/mL for each TG species. Nineteen TG molecular species were determined with an intra-day coefficient of variation of 15.0–20.9 % (n = 9), and an inter-day coefficient of variation of 17.3–36.6 % (n = 9). Recovery of TG 50:0 using the equivalent internal standard was 97.0 %. Nineteen TG molecular species can be analyzed in 2 min from human plasma or serum by the novel tandem mass spectrometric approach. In subsequent studies, the distribution of plasma TG molecular species can be analyzed under high-throughput conditions in healthy and diseased individuals.






Similar content being viewed by others
References
Storlien LH, Kriketos AD, Calvert GD, Baur LA, Jenkins AB (1997) Fatty acids, triglycerides and syndromes of insulin resistance. Prostaglandins Leukot Essent Fatty Acids 57(4–5):379–385. doi:10.1016/S0952-3278(97)90414-2
Nordestgaard BG, Benn M, Schnohr P, Tybjærg-Hansen A (2007) Nonfasting triglycerides and risk of myocardial infarction, ischemic heart disease, and death in men and women. JAMA 298(3):299–308
Han X, Gross RW (2001) Quantitative Analysis and Molecular Species Fingerprinting of Triacylglyceride Molecular Species Directly from Lipid Extracts of Biological Samples by Electrospray Ionization Tandem Mass Spectrometry. Anal Biochem 295(1):88–100. doi:10.1006/abio.2001.5178
Freiberg JJ, Tybjærg-Hansen A, Jensen J, Nordestgaard BG (2008) NOnfasting triglycerides and risk of ischemic stroke in the general population. JAMA 300(18):2142–2152
Rhee EP, Cheng S, Larson MG, Walford GA, Lewis GD, McCabe E, Yang E, Farrell L, Fox CS, O’Donnell CJ, Carr SA, Vasan RS, Florez JC, Clish CB, Wang TJ, Gerszten RE (2011) Lipid profiling identifies a triacylglycerol signature of insulin resistance and improves diabetes prediction in humans. J Clin Investig 121(4):1402–1411. doi:10.1172/jci44442
McBride PE (2007) Triglycerides and risk for coronary heart disease. JAMA 298(3):336–338
Hunter JE (2001) Studies on effects of dietary fatty acids as related to their position on triglycerides. Lipids 36(7):655–668. doi:10.1007/s11745-001-0770-0
Krank J, Murphy RC, Barkley RM, Duchoslav E, McAnoy A (2007) Qualitative analysis and quantitative assessment of changes in neutral glycerol lipid molecular species within cells. In: Brown HA (ed) Methods in enzymology, vol 432. Academic Press, USA, pp 1–20. doi:10.1016/S0076-6879(07)32001-6
Fernandez C, Sandin M, Sampaio JL, Almgren P, Narkiewicz K, Hoffmann M, Hedner T, Wahlstrand B, Simons K, Shevchenko A, James P, Melander O (2013) Plasma Lipid Composition and Risk of Developing Cardiovascular Disease. PLoS ONE 8(8):e71846
Schwudke D, Oegema J, Burton L, Entchev E, Hannich JT, Ejsing CS, Kurzchalia T, Shevchenko A (2005) Lipid Profiling by Multiple Precursor and Neutral Loss Scanning Driven by the Data-Dependent Acquisition. Anal Chem 78(2):585–595. doi:10.1021/ac051605m
Hsu F-F, Turk J (2010) Electrospray ionization multiple-stage linear ion-trap mass spectrometry for structural elucidation of triacylglycerols: assignment of fatty acyl groups on the glycerol backbone and location of double bonds. J Am Soc Mass Spectrom 21(4):657–669. doi:10.1016/j.jasms.2010.01.007
Myher JJ, Kuksis A, Marai L, Sandra P (1988) Identification of the more complex triacylglycerols in bovine milk fat by gas chromatography—mass spectrometry using polar capillary columns. J Chromatogr A 452:93–118. doi:10.1016/S0021-9673(01)81440-0
Hoving EB, Jansen G, Volmer M, van Doormaal JJ, Muskiet FAJ (1988) Profiling of plasma cholesterol ester and triglyceride fatty acids as their methyl esters by capillary gas chromatography, preceded by a rapid aminopropyl-silica column chromatographic separation of lipid classes. J Chromatogr B Biomed Sci Appl 434(2):395–409. doi:10.1016/S0378-4347(88)80006-9
McAnoy AM, Wu CC, Murphy RC (2005) Direct qualitative analysis of triacylglycerols by electrospray mass spectrometry using a linear ion trap. J Am Soc Mass Spectrom 16(9):1498–1509. doi:10.1016/j.jasms.2005.04.017
Bligh EG, Dyer WJ (1959) A rapid method for total lipid extraction and purification. Can J Biochem Physiol 37:911–917
Folch J, Lees M, Stanley GHS (1957) A simple method for the isolation and purification of total lipides from animal tissue. J Biol Chem 226:497–509
http://webbook.nist.gov. Accessed 15 April 2014
Fliszar KA, Peter Wuelfing W, Li Z, Reed RA (2006) Profiling of medium chain glycerides used in pharmaceutical formulation development by reversed-phase HPLC. J Pharm Biomed Anal 40(4):896–900. doi:10.1016/j.jpba.2005.08.024
Holčapek M, Dvořáková H, Lísa M, Girón AJ, Sandra P, Cvačka J (2010) Regioisomeric analysis of triacylglycerols using silver-ion liquid chromatography–atmospheric pressure chemical ionization mass spectrometry: Comparison of five different mass analyzers. J Chromatogr A 1217(52):8186–8194. doi:10.1016/j.chroma.2010.10.064
Dillon JT, Aponte JC, Tarozo R, Huang Y (2012) Efficient liquid chromatographic analysis of mono-, di-, and triglycerols using silver thiolate stationary phase. J Chromatogr A 1240:90–95. doi:10.1016/j.chroma.2012.03.083
Leiker TJ, Barkley RM, Murphy RC (2011) Analysis of diacylglycerol molecular species in cellular lipid extracts by normal-phase LC-electrospray mass spectrometry. Int J Mass Spectrom 305(2–3):103–108. doi:10.1016/j.ijms.2010.09.008
Hutchins PM, Barkley RM, Murphy RC (2008) Separation of cellular nonpolar neutral lipids by normal-phase chromatography and analysis by electrospray ionization mass spectrometry. J Lipid Res 49(4):804–813. doi:10.1194/jlr.M700521-JLR200
Nagy K, Sandoz L, Destaillats F, Schafer O (2012) Mapping the regioisomeric distribution of fatty acids in triacylglycerols by hybrid mass spectrometry. J Lipid Res. doi:10.1194/jlr.D031484
Nagy K, Sandoz L, Destaillats F, Schafer O (2013) Mapping the regioisomeric distribution of fatty acids in triacylglycerols by hybrid mass spectrometry. J Lipid Res 54(1):290–305. doi:10.1194/jlr.D031484
Lembcke J, Ceglarek U, Fiedler GM, Baumann S, Leichtle A, Thiery J (2005) Rapid quantification of free and esterified phytosterols in human serum using APPI-LC-MS/MS. J Lipid Res 46(1):21–26. doi:10.1194/jlr.C400004-JLR200
Quehenberger O, Armando AM, Brown AH, Milne SB, Myers DS, Merrill AH, Bandyopadhyay S, Jones KN, Kelly S, Shaner RL, Sullards CM, Wang E, Murphy RC, Barkley RM, Leiker TJ, Raetz CRH, Guan Z, Laird GM, Six DA, Russell DW, McDonald JG, Subramaniam S, Fahy E, Dennis EA (2010) Lipidomics reveals a remarkable diversity of lipids in human plasma. J Lipid Res 51(11):3299–3305. doi:10.1194/jlr.M009449
Roche_Diagnostics_GmbH (2011) Methodenbeschreibung Triglycerides für cobas c System
Liu L, Zhang Y, Chen N, Shi X, Tsang B, Yu Y-H (2007) Upregulation of myocellular DGAT1 augments triglyceride synthesis in skeletal muscle and protects against fat-induced insulin resistance. J Clin Investig 117(6):1679–1689. doi:10.1172/jci30565
Author information
Authors and Affiliations
Corresponding author
Additional information
Published in the topical collection Recent Developments in Clinical Omics with guest editors Martin Giera and Manfred Wuhrer.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Sander, M., Becker, S., Thiery, J. et al. Simultaneous Identification and Quantification of Triacyglycerol Species in Human Plasma by Flow-Injection Electrospray Ionization Tandem Mass Spectrometry. Chromatographia 78, 435–443 (2015). https://doi.org/10.1007/s10337-014-2782-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10337-014-2782-x