Abstract
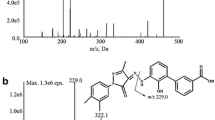
1-[(2-Chlorophenyl)diphenylmethyl]pyrazole (TRAM-34) is a highly selective KCa3.1 channel blocker. TRAM-34 was commonly used to study the role of KCa3.1 in the pathogenesis of disease in vivo, but there was no validated analytical method. Here, we describe the first validated LC–MS/MS analytical method for TRAM-34. Solid-phase extraction (SPE) was performed to extract TRAM-34 from the rat plasma. Chromatographic separation was achieved on the phenyl column. A triple quadrupole mass spectrometer was operated in positive-mode electrospray ionization. There were two multiple-reaction monitoring (MRM) transitions for TRAM-34: m/z 277.2 → 165.1 (for quantification) and m/z 277.2 → 241.2 (for qualification). Bifonazole was used as an internal standard. The lower limit of quantification (LLOQ) achieved was 1 ng mL−1 and the run time was 7.5 min. The linear range was from 1 to 1,000 ng mL−1. The pharmacokinetics profile was acquired for rats following an intraperitoneal injection of TRAM-34, with the following pharmacokinetics parameters found: C max 17.03 ± 1.34 ng mL−1; T max 8.67 ± 3.06 h; and T 1/2 13.45 ± 2.72 h. In addition, a suspected metabolite of TRAM-34 was found using this LC–MS/MS method. Given the results of the detailed validation process and its application to TRAM-34 pharmacokinetics, it is clear that a fast, selective, precise, and reproducible TRAM-34 LC–MS/MS analytical method was successfully established.





Similar content being viewed by others
References
Al-Ghananeem AM, Abbassi M, Shrestha S, Raman G, Wulff H, Pereira L, Ansari A (2010) Formulation-based approach to support early drug discovery and development efforts: a case study with enteric microencapsulation dosage form development for a triarylmethane derivative TRAM-34; a novel potential immunosuppressant. Drug Dev Ind Pharm 36(5):563–569. doi:10.3109/03639040903329554
Wulff H, Miller MJ, Hansel W, Grissmer S, Cahalan MD, Chandy KG (2000) Design of a potent and selective inhibitor of the intermediate-conductance Ca2+ -activated K+ channel, IKCa1: a potential immunosuppressant. Proc Natl Acad Sci USA 97(14):8151–8156
Toyama K, Wulff H, Chandy KG, Azam P, Raman G, Saito T, Fujiwara Y, Mattson DL, Das S, Melvin JE, Pratt PF, Hatoum OA, Gutterman DD, Harder DR, Miura H (2008) The intermediate-conductance calcium-activated potassium channel KCa3.1 contributes to atherogenesis in mice and humans. J Clin Invest 118(9):3025–3037. doi:10.1172/JCI30836
Kaushal V, Koeberle PD, Wang Y, Schlichter LC (2007) The Ca2+ -activated K+ channel KCNN4/KCa3.1 contributes to microglia activation and nitric oxide-dependent neurodegeneration. J Neurosci 27(1):234–244. doi:10.1523/JNEUROSCI.3593-06.2007
Mene P, Pirozzi N (2010) Potassium channels: the ‘master switch’ of renal fibrosis? Nephrol Dial Transplant 25(2):353–355. doi:10.1093/ndt/gfp634
Grgic I, Kiss E, Kaistha BP, Busch C, Kloss M, Sautter J, Muller A, Kaistha A, Schmidt C, Raman G, Wulff H, Strutz F, Grone HJ, Kohler R, Hoyer J (2009) Renal fibrosis is attenuated by targeted disruption of KCa3.1 potassium channels. Proc Natl Acad Sci USA 106(34):14518–14523. doi:10.1073/pnas.0903458106
Grgic I, Eichler I, Heinau P, Si H, Brakemeier S, Hoyer J, Kohler R (2005) Selective blockade of the intermediate-conductance Ca2+ -activated K+ channel suppresses proliferation of microvascular and macrovascular endothelial cells and angiogenesis in vivo. Arterioscler Thromb Vasc Biol 25(4):704–709. doi:10.1161/01.ATV.0000156399.12787.5c
Albaqumi M, Srivastava S, Li Z, Zhdnova O, Wulff H, Itani O, Wallace DP, Skolnik EY (2008) KCa3.1 potassium channels are critical for cAMP-dependent chloride secretion and cyst growth in autosomal-dominant polycystic kidney disease. Kidney Int 74(6):740–749. doi:10.1038/ki.2008.246
Jager H, Dreker T, Buck A, Giehl K, Gress T, Grissmer S (2004) Blockage of intermediate-conductance Ca2+ -activated K+ channels inhibit human pancreatic cancer cell growth in vitro. Mol Pharmacol 65(3):630–638. doi:10.1124/mol.65.3.630
Schmidt J, Friebel K, Schonherr R, Coppolino MG, Bosserhoff AK (2010) Migration-associated secretion of melanoma inhibitory activity at the cell rear is supported by KCa3.1 potassium channels. Cell Res 20(11):1224–1238. doi:10.1038/cr.2010.121
Lam J, Wulff H (2011) The lymphocyte potassium channels Kv1.3 and KCa3.1 as targets for immunosuppression. Drug Dev Res 72(7):573–584. doi:10.1002/ddr.20467
Wulff H, Gutman GA, Cahalan MD, Chandy KG (2001) Delineation of the clotrimazole/TRAM-34 binding site on the intermediate conductance calcium-activated potassium channel, IKCa1. J Biol Chem 276(34):32040–32045. doi:10.1074/jbc.M105231200
Reich EP, Cui L, Yang L, Pugliese-Sivo C, Golovko A, Petro M, Vassileva G, Chu I, Nomeir AA, Zhang LK, Liang X, Kozlowski JA, Narula SK, Zavodny PJ, Chou CC (2005) Blocking ion channel KCNN4 alleviates the symptoms of experimental autoimmune encephalomyelitis in mice. Eur J Immunol 35(4):1027–1036. doi:10.1002/eji.200425954
Guidance for industry, bioanalytical of health and human services, food and drug administration (2001) Available: http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM070107.pdf. Accessed May 2001
Matuszewski BK, Constanzer ML, Chavez-Eng CM (2003) Strategies for the assessment of matrix effect in quantitative bioanalytical methods based on HPLC-MS/MS. Anal Chem 75(13):3019–3030
Zgola-Grzeskowiak A, Grzeskowiak T (2011) The use of a triple quadrupole linear ion trap mass spectrometer with electrospray ionisation for fragmentation studies of selected antifungal drugs. Rapid Commun Mass Spectrom 25(20):3049–3055. doi:10.1002/rcm.5188
Weinmann W, Stoertzel M, Vogt S, Wendt J (2001) Tune compounds for electrospray ionisation/in-source collision-induced dissociation with mass spectral library searching. J Chromatogr A 926(1):199–209
Trufelli H, Palma P, Famiglini G, Cappiello A (2011) An overview of matrix effects in liquid chromatography-mass spectrometry. Mass Spectrom Rev 30(3):491–509. doi:10.1002/mas.20298
Little JL, Wempe MF, Buchanan CM (2006) Liquid chromatography-mass spectrometry/mass spectrometry method development for drug metabolism studies: examining lipid matrix ionization effects in plasma. J Chromatogr B 833(2):219–230. doi:10.1016/j.jchromb.2006.02.011
Chen YJ, Raman G, Bodendiek S, O’Donnell ME, Wulff H (2011) The KCa3.1 blocker TRAM-34 reduces infarction and neurological deficit in a rat model of ischemia/reperfusion stroke. J Cereb Blood Flow Metab 31(12):2363–2374. doi:10.1038/jcbfm.2011.101
Brugnara C, Armsby CC, Sakamoto M, Rifai N, Alper SL, Platt O (1995) Oral administration of clotrimazole and blockade of human erythrocyte Ca(++)-activated K+ channel: the imidazole ring is not required for inhibitory activity. J Pharmacol Exp Ther 273(1):266–272
Acknowledgments
We acknowledge Mr. Pei-Wei Huang, Senior Assistant, School of Pharmacy, National Defense Medical Center, for his technical support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ye, JH., Pan, RN., Guo, CC. et al. LC–MS/MS Method for Detection of a Highly Selective KCa3.1 Blocker, TRAM-34, in Rat Plasma. Chromatographia 77, 695–705 (2014). https://doi.org/10.1007/s10337-014-2674-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10337-014-2674-0