Abstract
Understanding the mechanisms and the factors influencing the phenology, reproduction and biometrics of long-distance migrants are extremely important as global climate warming has induced changes in the locations of geographic ranges and dates of migration and reproduction. In this article, we compare phenology, reproductive parameters and adult biometrics of core and peripheral populations of a rare and endangered bird—the barred warbler Curruca nisoria—that inhabits the farming landscapes and makes long-distance trans-continental migrations. We predicted that: (i) individuals nesting in the core population would be larger than in the peripheral population; (ii) the reproductive parameters of the Barred Warblers in the core population would be higher than in the peripheral population. The Barred Warblers nesting in the centre of the range laid larger clutches and were clearly larger than individuals from the small, peripheral population. However, no differences in egg size and productivity were observed between the analysed populations. The Barred Warblers synchronised their breeding dates over a large geographical area as they had only a short time window for reproduction and had to lay their eggs as soon as possible. Individuals arriving earlier on the breeding grounds had larger clutches, longer wings and produced more nestlings. The pressure of the passage of time during the breeding season was a more important factor than the geographical location affecting the breeding characteristics in these populations.
Zusammenfassung
Zeitlicher Verlauf, Fortpflanzung und biometrische Daten eines langstreckenziehenden Singvogels in einer Kern- und einer Randpopulation
Das Verständnis der Mechanismen und Faktoren, welche die Phänologie, Fortpflanzung und biometrischen Daten von Langstreckenziehern beeinflussen, ist äußerst wichtig, da der globale Klimawandel zu Veränderungen der geographischen Verbreitungsgebiete und Zug- und Fortpflanzungszeiten geführt hat. In dieser Studie vergleichen wir die Phänologie, Fortpflanzungsparameter und biometrischen Daten von adulten Individuen einer im Verbreitungsgebiet zentral gelegenen Kernpopulation und einer peripher gelegenen Randpopulation einer seltenen und gefährdeteren Vogelart—der Sperbergrasmücke Curruca nisoria—welche die Agrarlandschaften bewohnt und transkontinentale Langstreckenflüge vollzieht. Wir stellten die Hypothesen auf, dass: (i) Individuen, die in der Kernpopulation brüten, größer sein würden als in der Randpopulation; (ii) die Fortpflanzungsparameter der Sperbergrasmücke in der Kernpopulation höher sein würden als in der Randpopulation. Sperbergrasmücken, die im Zentrum des Verbreitungsgebietes brüteten, legten größere Gelege und waren deutlich größer als die Individuen der kleinen Randpopulation. Jedoch konnten wir keine Unterschiede in der Eigröße und in der Produktivität zwischen den beiden untersuchten Populationen beobachten. Die Sperbergrasmücke synchronisierte ihren Brutzeitpunkt über ein großes geographisches Areal, da sie nur ein kurzes Zeitfenster für die Fortpflanzung hat und Eier so schnell wie möglich legen muss. Individuen, die früher in den Brutgebieten eintrafen, hatten größere Gelege, längere Flügel und mehr Küken. Der zeitliche Druck während der Brutsaison beeinflusste die Bruteigenschaften in diesen Populationen mehr als die geografische Lage.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
A fundamental aim of macroecology is to understand the impact of different geographical and climatic conditions on the distribution pattern and abundance of organisms (Doherty et al. 2003). Every species has its own characteristic and limited distribution range (Geber 2011). However, organisms occurring over large areas clearly differ in genetic and phenotypic characteristics (Meissner and Krupa 2007; Summers et al. 2009; Sokolovskis et al. 2019), phenology (Sanz 1998), reproductive parameters (Garcia and Arroyo 2001; McGowan et al. 2005) and mortality rates (Sergio et al. 2019). Populations located in the centre of their range usually inhabit optimal and productive habitats, which lie in the climatic optimum specific to each species (Bridle and Vines 2006). They are referred to by naturalists as source populations, in which emigration of individuals predominates over their immigration (Dias 1996). Peripheral populations are referred to as sink populations, where mortality exceeds birth rates and are usually supplied with individuals from source populations (Lawton 1996).
In comparison with smaller, peripheral populations of a species living at the edge of its range, avian populations inhabiting the centre of their geographical ranges are characterised by higher abundance and density (Lawton 1996; McGowan et al. 2005; Soutullo et al. 2006), earlier breeding initiation dates (Sanz 1998; Tryjanowski et al. 2006, but this also depends on latitude—see e.g. Arslan et al. 2016; Söderström and Karlsson 2011), higher breeding success (Tryjanowski et al. 2006; Lohr et al. 2011) and a lower risk of extinction (Bridle and Vines 2006; Lohr et al. 2011).
In recent years, there has been many discussions and controversies amongst conservationists about the priorities and focus on conservation efforts to maintain populations of rare and endangered animal species (Goławski et al. 2016; Boakes et al. 2018; Oldfather et al. 2020). Some researchers consider that the focus should be on protecting peripheral and small populations, as they are more vulnerable to extinction (Vucetich and Waite 2003). In contrast, other experts suggest that the main efforts and conservation actions should concentrate on source populations, from which there is migration of individuals both to new areas and to suboptimal habitats at the edge of the range (Soutullo et al. 2006; Lohr et al. 2011). This is more important because in recent years, global climate warming has been causing many of these species to change their geographical distribution, phenology, migration routes and location of wintering grounds (Oldfather et al. 2020). These differing suggestions show that it is critical to understand evolutionary and ecological mechanisms that shape demographic and genetic processes at large spatial scales (Lewis et al. 2012; Tøttrup et al. 2012).
Birds migrating between different continents have particularly large and fragmented geographical ranges (Laton 1996; Meissner and Krupa 2007). The simultaneous laying of eggs by females is particularly important in the case of migrants making long-distance journeys between their wintering and breeding grounds (Polak 2015). In the temperate climate zone, migratory birds usually have only a short period in which to reproduce, so they are under strong pressure of time (Tøttrup et al. 2012). Thus, individuals nesting later in the breeding season generally produce either smaller broods or poor quality offspring (Wiklund 1984; Borgmann et al. 2013). Understanding the mechanisms and the factors influencing the phenology of long-distance migrants is all the more important as, in recent years, the warming climate has given rise to changes in the geographical ranges and the periods of migration and reproduction in many avian populations (Garcia and Arroyo 2001; Soutullo et al. 2006; Lohr et al. 2011).
Here, we compare the nesting phenology, reproductive parameters and biometrics of a rare and endangered bird—the barred warbler Curruca nisoria—which inhabits agricultural landscapes (Goławski 2007; Pestka et al. 2018; Polak 2019) and makes long-distance transcontinental migrations (Hedenström et al. 2022). Our previous work has shown that this species is under strong pressure of time to breed as early as possible (Polak 2015, 2016). We compared populations located in the centre and at the edge of its geographical range in an attempt to find a source-sink gradient and/or latitudinal pattern in this system. We formulated and tested the following initial hypotheses: (i) as a result of nesting in more productive and optimal habitats, the reproductive parameters of the barred warbler in a population living in the centre of its range will be higher than in a population inhabiting areas at the edge of its range; (ii) adults nesting in the core (source) population will be larger and in better condition than those in the peripheral (sink) population.
Methods
Study species
The barred warbler is a small passerine from the family Sylviidae, weighing 21–28 g, whose range is restricted to central and eastern Europe, and also Asia (Kazakhstan, Mongolia, and China) (Neuschulz 1988; Cramp 1992; Goławski 2007). It builds very well-hidden, basket-shaped nests from plant materials and animal hair (Polak 2012, 2019; Orłowski et al. 2015). Four to seven eggs are laid and are incubated for 14 days (Neuschulz 1981; Polak 2016). This species is normally single-brooded, but in case of first brood failure, replacement clutches are laid regularly (Polak 2015). The barred warbler is a trans-continental migrant wintering in central and eastern Africa (Cramp 1992; Hedenström et al. 2022). It still has high protection status in Europe, being listed in Annex 1 of The Birds Directive on the conservation of wild birds (Directive 2009/147/EC) (Polak and Filipiuk 2014). The main threat to the Barred Warbler’s population in Europe is the continuing reduction of habitat availability as a consequence of the destruction or deterioration of farmland habitats (Donald et al. 2001; Polak 2012; Pestka et al. 2018).
Study plots
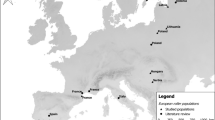
The field work was carried out on two study plots in eastern Poland (the core of the Barred Warbler’s breeding range) and on one plot in the southern Czech Republic (the periphery of its breeding range).
Stężyca
Fieldwork on this study plot, near the village of Stężyca (N 51°34'; E 21°48') in eastern Poland, the research was conducted in 2009–2011 (Polak 2013). This plot, 76 ha in area (84 ha in 2010), was a well-isolated, dry pasture in the Middle Vistula valley. Reduced grazing by cattle and horses in recent years has since led to rapid succession to shrubby vegetation, especially the Hawthorns Crataegus sp. To the east, the plot was bounded by an ox-bow, to the west by the River Vistula. There were small patches of riverine willow-poplar woodland Salici-populetum. The fieldwork on this plot embraced a mosaic of four principle habitat types: open terrain (42.8%), shrubs and bushes (29.4%), trees (19.7%) and water (8.1%). The density of barred warblers varied from 2.2 to 3.2 pairs (territories)/10 ha (Polak 2012).
Żurawnica
This 106-ha study plot was situated in farmland near the village of Żurawnica (N 50°38'; E 22°58') in the Roztocze region of eastern Poland. The study fieldwork took place there in 2012–2015 (Polak 2016, 2019). Characteristic of this region are deforested hills, now covered by long, narrow strip fields, separated by high baulks. The main crops in this plot were cereals. The fieldwork here covered a mosaic of six principal habitat types: fields (65.9%), fallow land (22.3%), bushes (6.4%), trees (3.1%), roads (2.2%) and buildings (0.1%). The density of Barred Warblers varied from 1.9 to 3.2 pairs (territories)/10 ha (Polak and Filipiuk 2014).
Znojmo
This 130-ha study plot, where the fieldwork was carried out between 2012 and 2014, was located on an abandoned military training area near the town of Znojmo in the Czech Republic (South Moravia; N 48°49'; E 16°05'). This was the “Načeratický Kopec”, a hill in a lowland landscape with a maximum elevation of 290 m above sea level. It was an open steppe grassland habitat that had started to become overgrown with thorny shrubs (especially dog rose and hawthorn) and low trees. The density of barred warblers was 4 pairs/10 ha.
Methods
Data collection
From early May to mid–July, the plots were surveyed regularly every few days to discover as many barred warbler territories and nests as possible. The basic method of locating nests was a systematic search of all potential breeding sites in the shrubs growing on the study plots. Nests were checked at any time of the day in calm dry weather. The position of each nest was marked on an orthophotograph, and the exact coordinates were entered on a GPS receiver. Nests were inspected in order to establish basic reproductive parameters, i.e. egg-laying date, clutch size, hatching date and number of nestlings (Polak 2014, 2016, 2019). The length and the width of the eggs were measured with callipers to an accuracy of 0.1 mm. Because barred warbler eggs resemble those of the red-backed shrike (Lanius collurio) in shape and size, we calculated the volume of the former using the equation for the egg volume of the latter: volume = 0.5322 × length × (width)2 (Surmacki et al. 2006). Only eggs from complete clutches were analysed; replacement clutches were not taken into consideration. To avoid the problem of pseudoreplication, egg size was defined as the mean volume of all eggs laid in each nest (Zduniak and Antczak 2003). During each nest inspection, the numbers of nestlings were noted and biometric measurements taken, i.e. wing length and body mass to determine the age of the young birds. These two parameters appear to be best suited for describing the growth of barred warbler nestlings (our unpublished data; Neuschulz 1988). Clutch initiation dates were defined as the day the first egg was laid. Laying dates were determined from direct observations of incomplete clutches found during the laying process in the early breeding season as revealed by the greater number of eggs during the next visit or indirectly by back-calculating the hatching date of the oldest nestling, assuming that: (1) the incubation period lasts 14 days, (2) one egg is laid per day, (3) the female starts incubating after laying the penultimate egg (Cramp 1992; our unpublished data), and (4) the nestling age corresponds to the stages and growth rates given in Neuschulz (1988). First egg-laying dates were calculated as absolute (calendar) dates expressed by the number of days since 1 May. To avoid the problems with pseudo-replication of the clutches the same, marked birds in subsequent years were excluded from the analyses.
A nest was considered to be a replacement clutch if a clutch had failed in a territory and the second brood was later initiated by the parents close by. As stated above, replacement clutches were not analysed in this research. Data from a total of 98 barred warbler nests were thus worked up. Nests were considered successful when at least one juvenile fledged. The number of fledglings per breeding pair was defined as the number of young reaching 12–14 days of age in all the broods examined in the given population, and the number of fledglings per successful pair was defined as the number of young reaching 12–14 days of age in successful pairs (when at least one young bird had survived to 12–14 days old).
A total of 191 adult barred warblers were caught near active nests using mist nets and playback. We tried to trap the birds immediately after their arrival, but in some cases, it was not possible (especially for some females). In our populations, we found that the birds arrived on the breeding grounds in several waves. The first wave of birds arrived in early May and was later joined by further batches of migrants (Polak 2015). The parents clearly responded to vocal stimulation at the beginning of the breeding season when territory boundaries were being established, but thereafter their activity gradually decreased. The males were captured between 30 April and 3 July (mean = 21 May; SD = 10.9 days; n = 144), the females between 6 May and 4 July (mean = 25 May; SD = 12.9 days; n = 47). Each bird was individually marked with metal and coloured rings. Age and sex of the captured barred warblers were determined on the basis Svensson (1992) and Demongin (2016). Measurements were taken according to Svensson (1992) and Baker (1993). A ruler was used for measuring wing lengths (± 1 mm) and callipers for measuring tarsus length (± 0.1 mm) and head length (± 0.1 mm). The adults were weighed with a Pesola spring balance (± 0.5 g).
Statistical analyses
The mean and the standard deviation (± SD) of the biometrics and breeding characteristics were calculated. The differences in breeding phenology between these three local populations were analysed using medians, means and the Kruskal–Wallis test. The calculations were made in STATISTICA 13.3 packet for Windows software (StatSoft Inc. 2021). To test the effects of various factors on reproductive and biometric characteristics, general linear models (GLMs) were used. In all models, the first egg-laying date (1 being May 1) and the Julian capture date (1 being January 1) were continuous predictors, and sex and site were included as categorical factors. A Gaussian GLM with an identity link function was used to assess the impact of location and the date of laying the first egg on clutch size, mean egg volume and the number of fledglings per successful pair. Site was considered as a categorical factor and laying date as a continuous factor. To investigate the influence of laying date (a continuous predictor), sex, and site (categorical predictors) on egg size and biometric parameters, a Gaussian GLM with an default identity link function was utilised. The calculations by the use of general linear models (GLMs) were performed in the R Studio 2023.09.0 environment using R language version 4.3.1 (R Core Team 2023) and libraries: lme4, tidyverse.
Results
Nesting phenology
The earliest first egg-laying date was recorded in Znojmo on 5 May, whilst laying in Stężyca began a little later on 8 May, and later still in Żurawnica on 14 May. Nevertheless, most females laid their first eggs at a similar time in late May in all three localities, and this parameter did not differ statistically amongst the three study areas (Kruskal–Wallis test; H 2, 93 = 2.1; P = 0.346). The median first egg-laying date was 23 May in Znojmo (n = 25), 20 May in Stężyca (n = 31), and 24 May in Żurawnica (n = 37). The earliest hatching of the first chick was recorded in Znojmo on 21 May, then in Stężyca on 24 May, and finally in Żurawnica on 30 May. In all three populations, most of the young hatched in early and mid-June. The median hatching date of in Stężyca (n = 22) and in Znojmo (n = 20) was the same, i.e. 8 June and 11 June in Żurawnica (n = 31) (Kruskal–Wallis test; H 2, 73 = 6.1; P = 0.05).
Breeding ecology
The average number of eggs in a clutch was 4.9 ± 0.6 (n = 87; range 3–6; CV = 12.9%; Table 1) and the modal clutch was five (74% of total). During all the years of research, ten nests with six eggs were found: five in Stężyca (19% of all broods), four in Żurawnica (12%), but just one in Znojmo (4%). The clutch size was affected by the location of the study area and the first egg-laying date (Table 2). The females laid the largest clutches in the core part of breeding range in Poland, whilst in the edge of the range in Czech, they were significantly smaller. The number of eggs in the clutch significantly decreased with the progress of the breeding season. Egg size was not affected by either the location of the study area or the breeding onset date (Table 2). Forty-eight of the 96 broods were successful (50%; the fate of two broods was unknown). The number of fledglings per successful nest ranged from 2 to 6 (mean = 4.3 ± 0.9; CV = 12.6%; n = 49). The production of young in all broods was similar in all three locations (Table 1) and was significantly dependent on the clutch initiation date (Table 2).
Biometrics
Males had longer wings than females (Table 3). Wing length was significantly influenced by: sex, location and date of capturing (Table 4). The birds had longer wings in the core part of the Barred Warbler’s breeding range than on its periphery (Table 3). Birds caught in the second half of the season had shorter wings than at the beginning of the breeding period. Body weight depended on location and sex (Table 4), but there was no relationship between body weight and the date of capture. Females were significantly heavier than males (Table 3). Birds from the core part of the nesting range were heavier than in the peripheral. Tarsus length and head size were dependent on the location of the study area, whereas sex and capture date were not significant (Table 4). The values of the former two biometric parameters were significantly higher in the birds from Stężyca and Żurawnica than in those from the Czech Republic (Table 3).
Discussion
The results of this study only partially confirmed the initial prediction that birds from core (and also more northern) populations should have higher reproductive parameters. Barred warblers breeding in the centre of the range laid more eggs on average than in the peripheral population. There were no significant differences in egg size or in fledgling productivity amongst the analysed populations. However, this investigation shows that the productivity of the barred warbler was strongly influenced by the progress of the breeding season. It confirms previous observations that late breeders have lower fledgling productivity (Polak 2015, 2016; Bažant M. unpublished data).
Our earlier analyses showed that, owing to the narrow time window, barred warbler populations were under strong selection pressure to breed as early as possible in a strong manner (Polak 2015). It may be that individuals have to synchronise their breeding dates over a large geographical area. This barred warbler is a long-distance migrant wintering in eastern Africa (Hedenström et al. 2022) and arrives on its breeding grounds in Europe late in early May (Neuschulz 1981; Polak 2015). The present study showed that the birds started laying first eggs at the same time both in the centre and at the edge of their range. Transcontinental migrants arrive en masse on the breeding grounds and appear on the same day over large geographical areas (Tøttrup et al. 2012). The autumn migration of barred warbler also begins early. They depart for their wintering grounds as early as August (Neuschulz 1981; Cramp 1992). Adult birds thus stay for less than four months on the breeding grounds (Neuschulz 1988; Hedenström et al. 2022). Consequently, only a short time window is available for reproduction, so the birds have to lay their eggs as soon as possible (Polak 2013). The precise synchronisation of the first egg-laying dates over a large geographical area may be a consequence the fact that after their spring arrival, these birds are under pressure of time to start breeding as soon as possible (Polak 2015).
The results of this study however should be treated with caution. The differences in nesting ecology between the populations could be due to a number of factors not taken into consideration in these analyses. There is often considerable inter-annual variability in the breeding parameters and timing of reproduction in birds (Polak 2015, 2016). A further factor distorting our picture could have been the confounding of the location effect (centre vs edge) with the latitude effect.
To the best of our knowledge, this is the first research that has attempted to analyse the biometric dimensions of the barred warbler on the basis of a large sample. It showed that the adult birds in the centre of the breeding range were clearly larger than in the peripheral population and that there were significant differences in all biometric parameters. There may be various reasons for this general pattern (Meissner and Krupa 2007; McLoughlin et al. 2012; Matyjasiak et al. 2022). One is that the centre of the species’ range is inhabited by birds in better genetic and phenotypic conditions (Geber 2011; Ponce-Reyes et al. 2014; Demongin 2016). They occupy abundant and productive habitats with rich food resources (Vucetich and Waite 2003; Soutullo et al. 2006). Some avian biometrics are strongly correlated with body conditions, one of them is the tarsus length, which increases to its final value whilst the bird is still in the nest and never changes thereafter (Green 2001). It is a parameter strongly influenced by the rate of feeding and the type of food ingested during the nestling period, a critical period when all the organs, bones and tissues are developing (Grant 1971; Lindström 1999; Demongin 2016). Barred warbler nestlings are fed different food, depending on their age (Cramp 1992). Younger nestlings are fed soft, energetically rich food containing little chitin, whereas older nestlings receive more food of lower quality containing many chitin parts (Orłowski et al. 2015). The second reason for the biometric variation pattern found in birds analysed here could be the variation in size according to latitude (Cramp 1992; Meissner and Krupa 2007; McLoughlin et al. 2012). The Polish populations in the centre of the range lived further north than the peripheral population from the Czech Republic, and this factor could have influenced the adult biometrics. According to Bergmann's rule, individuals of warm-blooded species from northern populations, living in colder climates, are larger than their southern counterparts (Yom-Tov 2001). Moreover, long-distance migrants inhabiting the northern edge of their range have longer and more pointed wings, as this makes it easier for them to migrate along longer flyways between the breeding and the wintering grounds (Matyjasiak et al. 2022).
The present study revealed little sexual dimorphism amongst adult barred warblers, but there were significant differences in two biometric parameters—wing length and body mass. In all three populations, males had significantly longer wings than the females, but tarsus and head lengths were similar. The greater body mass amongst the females was due to their larger fat reserves for future egg formation and/or presence of eggs in the reproductive tracts.
This analysis also showed that males and females arriving earlier on the breeding grounds had longer wings compared to individuals captured later in the breeding season. When returning from the wintering grounds, it is important to arrive as early as possible in order to occupy the best and most productive territories (Tøttrup et al. 2012; Polak 2015). It is most likely that birds with long wings covered the long-distance migration route more efficiently and were the first to occupy optimal habitats (Matyjasiak et al. 2022), whereas the later arriving birds with shorter wings had no choice but to occupy sub-optimal nesting sites.
Conclusions
The barred warblers nesting in the centre of their range laid more eggs and were clearly larger than those from small, peripheral populations. There were no differences in the timing of breeding initiation. Birds arriving earlier on the breeding grounds had larger clutches, longer wings and produced more nestlings. The pressure of the passage of time during the breeding season was more important than geographical location as a factor affecting breeding parameters.
Data availability
Data available from the first author on reasonable request.
References
Arslan NS, Turan SL, Ayas Z (2016) Breeding biology of Red-backed Shrike, Lanius collurio, in the Kizilimark Delta in the north of Turkey. Turk J Zool 40:480–488. https://doi.org/10.3906/zoo-1509-8
Baker K (1993) Identification Guide to European Non-Passerines. BTO Guide 24. Thetford, BTO.
Boakes EH, Isaac NJB, Fuller RA, Mace GM, McGowan PJK (2018) Examining the relationship between local extinction risk and position in range. Conserv Biol 32:229–239. https://doi.org/10.1111/cobi.12979
Borgmann KL, Conway CJ, Morrison ML (2013) Breeding phenology of birds: mechanisms underlying seasonal declines in the risk of nest predation. PLoS One 8:e65909. https://doi.org/10.1371/journal.pone.0065909
Bridle JR, Vines TH (2006) Limits to evolution at range margins: when and why does adaptation fail? Trends Ecol Evol 22:140–147
Cramp S (1992) Handbook of the birds of Europe, the Middle East and North Africa. The birds of the western Palearctic vol VI: Warblers. Oxford, Oxford University Press.
Demongin L (2016) Identification guide to birds in the hand. Beauregard-Vendon.
Dias PC (1996) Sources and sinks in population biology. TREE 11:326–330
Doherty PF, Boulinier T, Nichols JD (2003) Local extinction and turnover rates at the edge and interior of species’ ranges. Ann Zool Fennici 40:145–153
Donald P, Green R, Heath M (2001) Agricultural intensification and the collapse of Europe’s farmland bird populations. Proc R Soc Lond Ser B Biol Sci 268:25–29. https://doi.org/10.1098/rspb.2000.1325
Garcia JT, Arroyo BE (2001) Effect of abiotic factors on reproduction in the centre and periphery of breeding ranges: a comparative analysis in sympatric harriers. Ecography 24:393–402
Geber MA (2011) Ecological and evolutionary limits to species geographic ranges. Am Nat 178:S1–S5. https://doi.org/10.1086/661899
Goławski A (2007) Does the Red–backed Shrike (Lanius collurio L.) benefits from nesting in the association with the Barred Warbler (Sylvia nisoria Bechst.)? Pol J Ecol 55:601–604
Goławski A, Mróz E, Kasprzykowski Z (2016) Breeding performance of the White-winged Tern (Chlidonias leucopterus) in two habitat types at the edge of their distribution range Ornis Fennica 93:225–234
Grant PR (1971) Variation in the tarsus length of birds in island and mainland regions. Evolution 25:599–614
Green AJ (2001) Mass/length residuals: measures of body condition or generators of spurious results? Evolution 82:1473–1483
Hedenström A, Åkesson S, Bensch S, Hasselquist D, Lindström Å (2022) Seasonally divided moult in the Barred Warbler (Sylvia nisoria) is an endogenously controlled strategy. Ibis 164:350–353. https://doi.org/10.1111/ibi.13001
Statsoft Inc. 2021. Statistica for Windows (data analysis system). Version 13.3.Statsoft Inc.
Lawton JH (1996) Population abundances, geographic ranges and conservation: 1994 Witherby Lecture. Bird Study 43:3–19. https://doi.org/10.1080/00063659609460991
Lewis S, Nussey DH, Wood AG, Croxall JP, Phillips RA (2012) Intrinsic determinants of a population trend in timing of breeding in the wandering albatross. Oikos 121:2061–2071. https://doi.org/10.1111/j.1600-0706.2012.20293.x
Lindström J (1999) Early development and fitness in birds and mammals. Trends Ecol Evol 14:343–348
Lohr M, Collins BM, Castelli PM, Williams CK (2011) Life on the edge: northern bobwhite ecology at the Northern periphery of their range. J Wildlife Manage 75:52–60. https://doi.org/10.1002/jwmg.25
Matyjasiak P, López-Calderón C, Ambrosini R, Balbontin J, Costanzo A, Kiat Y, Romano A, Rubolini D (2022) Wing morphology covaries with migration distance in a highly aerial insectivorous songbird. Curr Zool. https://doi.org/10.1093/cz/zoac044
McGowan CP, Simons TR, Golder W, Cordes J (2005) A Comparison of American Oystercatcher Reproductive Success on Barrier Beach and River Island Habitats in Coastal North Carolina. Waterbirds 28:150–155
McLoughlin DT, Beaubier JE, Sowter DJ, Corse CJ, Raine AF, Benson C, Cotton DC (2012) The biometrics of Twite Carduelis flavirostris in Ireland and Britain. Ringing Migr 27:76–82. https://doi.org/10.1080/03078698.2012.747644
Meissner W, Krupa R (2007) Biometrics and primary molt of common tern and sandwich tern in autumn in Puck Bay, Southern Baltic. Waterbirds 30:158–163. https://doi.org/10.1675/1524-4695
Neuschulz F (1981) Brutbiologie einer Population der Sperbergrasmücke (Sylvia nisoria) in Norddeutschland. J Ornithol 122:231–257
Neuschulz F (1988) Zur Synőkie von Sperbergrasmücke Sylvia nisoria (Bechst., 1975) und Neuntöter Lanius collurio (L., 1758). Luchow–Dannenberger Orn. Jber.
Oldfather MF, Kling MM, Sheth SN, Emery NC, Ackerly DD (2020) Range edges in heterogeneous landscapes: Integrating geographic scale and climate complexity into range dynamics. Global Change Biol 26:1055–1067. https://doi.org/10.1111/gcb.14897
Orłowski G, Wuczyński A, Karg J (2015) Effect of brood age on nestling diet and prey composition in a hedgerow specialist bird, the barred warbler Sylvia nisoria. PLoS ONE 10:1–16. https://doi.org/10.1371/journal.pone.0131100
Pestka Z, Jakubas D, Wojczulanis-Jakubas K (2018) Habitat preferences of Red-backed Shrikes Lanius collurio and Barred Warblers Sylvia nisoria breeding sympatrically in a wetland/farmland mosaic. Bird Study 65:317–328
Polak M (2012) Habitat preferences of the sympatric barred warbler (Sylvia nisoria) and the red–backed shrike (Lanius collurio) breeding in central Poland. Ann Zool Fenn 49:355–363. https://doi.org/10.5735/086.049.0509
Polak M (2013) Comparison of nest defence behaviour between two associate passerines. J Ethol 31:1–7. https://doi.org/10.1007/s10164-012-0340-2
Polak M (2014) Protective nesting association between the Barred Warbler Sylvia nisoria and the Red–backed Shrike Lanius collurio: an experiment using artificial and natural nests. Ecol Res 29:949–957. https://doi.org/10.1007/s11284-014-1183-9
Polak (2015) Variation in the nesting phenology of two positively interacting passerines. Avian Biol Res 8:1–7. https://doi.org/10.3184/175815515X14279001268462
Polak M (2016) Comparative breeding ecology, nest survival and agonistic behaviour between Barred Warbler and Red–backed Shrike. J Ornithol 157:747–758. https://doi.org/10.1007/s10336-016-1336-4
Polak M (2019) Nest concealment and nest defense by two passerines: effect of protective nesting association. Zool Stud 58:e15. https://doi.org/10.6620/ZS.2019.58-15
Polak M, Filipiuk M (2014) Habitat preferences of the barred warbler sylvia nisoria and the red–backed shrike lanius collurio in the Middle Roztocze region. Orn Pol 55:22–23 ((in Polish))
Ponce-Reyes R, Clegg SM, Carvalho SB, Mcdonald-Madden E, Possingham HP (2014) Geographical surrogates of genetic variation for selecting island populations for conservation. Divers Distrib 20:640–651. https://doi.org/10.1111/ddi.12195
R Core Team (2023) _R: A Language and Environment for Statistical Computing_. R Foundation for Statistical Computing, Vienna, Austria. <https://www.R-project.org/>.
Sanz JJ (1998) Effects of geographic location and habitat on breeding parameters of great tits. Auk 115:1034–1051
Sergio F, Tavecchia G, Tanferna A, Blas J, Blanco G, Hiraldo F (2019) When and where mortality occurs throughout the annual cycle changes with age in a migratory bird: individual vs population implications. Sci Rep 9:17352. https://doi.org/10.1038/s41598-019-54026-z
Söderström B, Karlsson H (2011) Increased reproductive performance of Red-backed Shrikes Lanius collurio in forest clear-cuts. J Ornithol 152:313–318
Sokolovskis K, Lundberg M, Liedvogel M, Solovyeva D, Åkesson S, Willemoes M, Bensch S (2019) Phenotypic and genetic characterization of the East Siberian Willow Warbler (Phylloscopus trochilus yakutensis Ticehurst, 1935) in relation to the European subspecies. J Ornithol 160:721–731
Soutullo A, Liminana R, Urios V, Surroca M, Gill JA (2006) Denisty-dependent regulation of population size in colonial breeders: alee and buffer effects in the migratory Montagu’s harrier. Oecologia 149:543–552
Summers R, Hallgrimsson G, Aiton D, Etheridge B, Heaton J, Swann B (2009) Population structure, biometrics and moult of migrant purple sandpipers Calidris maritima in southwest Iceland in spring. Bird Study 56:357–368
Surmacki A, Kuczyński L, Tryjanowski P (2006) Eggshell patterning in the red–backed Shrike Lanius collurio: relation to egg size and potential function. Acta Ornithol 41:145–151
Svensson L (1992) Identification guide to European Passerines, 4th edn. L. Svensson, Stockholm
Tøttrup AP, Klaassen RHG, Kristensen MW, Strandberg R, Vardanis Y, Lindström Å, Rahbek C, Alerstam T, Thorup K (2012) Drought in Africa caused delayed arrival of European songbirds. Science 338:1307–1307. https://doi.org/10.1126/science.1227548
Tryjanowski P, Sparks TH, Crick HQP (2006) Red-backed Shrike (Lanius collurio) nest performance in a declining British population: a comparison with a stable population in Poland. Ornis Fennica 83:181–186
Vucetich JA, Waite TA (2003) Spatial patterns of demography and genetic processes across the species’ range: Null hypotheses for landscape conservation genetics. Conserv Genet 4:639–645
Wiklund CG (1984) Reproductive synchrony in the fieldfare (Turdus pilaris) in relation to spring arrival, nest predation and nestling starvation. Behav Ecol Sociobiol 15:311–316
Yom-Tov Y (2001) Global warming and body mass decline in Israeli passerine birds. Proc R Soc Lond B 268:947–952
Zduniak P, Antczak M (2003) Repeatability and within-clutch varaiation in egg dimensions in a hooded crow Corvus corone cornix population. Biol Lett 40:37–42
Acknowledgements
We would like to thank Maciej Filipiuk, Jana Sýkorová, Markéta Buršíková and Václav Mikeš for their assistance in the field, and Peter Senn for the linguistic corrections. Special thanks to Emilia Łabuć for her advice and help with the statistics. We are grateful to the three anonymous referees for their helpful comments and suggestions for improving the manuscript.
Funding
This study was supported by the Maria Curie Skłodowska University in Lublin (Grant of Vice–Rector for Research and International Cooperation No. BW–01–1200–01–10) and the University of South Bohemia in České Budějovice (Grant GAJU 168/13/P).
Author information
Authors and Affiliations
Contributions
MP conceptualisation (equal); investigation (equal); methodology (equal); data collection (equal); data curation (equal); formal analysis (lead); validation (equal); visualisation (equal); writing-original draft (lead); writing-review and editing (lead). MB conceptualization (equal); investigation (equal); methodology (equal); validation (equal).
Corresponding author
Ethics declarations
Conflict of interest
The authors have no competing interests to declare that are relevant to the content of this article.
Ethical approval
This study complies with current Polish and Czech laws. The study was permitted by the Local Ethics Commission in Lublin (approval number: 5/2011). The Provincial Nature Conservator in Lublin approved for this research project (approval number: 165 WPN.6401.200.2012.PD).
Additional information
Communicated by F. Bairlein.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Polak, M., Bažant, M. Timing, reproduction and biometrics of a long-distance passerine migrant in a core and a peripheral population. J Ornithol 165, 405–413 (2024). https://doi.org/10.1007/s10336-023-02135-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10336-023-02135-y