Abstract
Habitat conditions surrounding individual birds are key components determining their fitness, notably by influencing effective foraging, escape from predators, and adaptation to changing environmental conditions. Birds living in dynamic habitats are particularly likely to have a high need to explore; however, spatial and temporal patterns of exploratory movements are poorly understood. We investigated the exploratory movements of adult common shelducks (hereafter Shelducks, Tadorna tadorna) in the Wadden Sea, which provides a dynamic habitat for birds. We captured nine shelducks in adjacent breeding areas and attached GPS satellite telemetry devices for 5–18 months, mostly covering the post-breeding period. We analyzed bird-movement patterns based on mean daily locations and behavioral change point analysis. All individuals explored on a median of 22% of tracking days (range = 6–39%). Individuals usually explored new areas for a short time before switching to stay in these areas for longer periods. Exploratory movements were particularly frequent before the start of the flightless period during molting. During this time, shelducks explored the traditional molting area in the Elbe estuary intensively. However, birds did not explore places within the area subsequently used for molting for longer or more often than other places. The molting area was used for a median of 59 days, i.e., about twice as long as required for the flightless period, suggesting that this area was explored not only to select a suitable molting place, but also for other purposes. Only two shelducks performed longer-distance exploration movements, i.e., > 45 km from their sedentary areas. We therefore concluded that exploratory movements by adult shelducks may enable the birds to adapt to small-scale changes in habitat conditions.
Zusammenfassung
Saisonale Erkundungsphasen bei adulten Brandgänsen im Wattenmeer
Lebensraumbedingungen sind von hoher Bedeutung für die Fitness einzelner Vögel durch Einflüsse auf eine effektive Nahrungssuche und Flucht vor Raubtieren. Vögel, die in dynamischen Lebensräumen leben, haben mit hoher Wahrscheinlichkeit einen hohen Bedarf darin, ihre Umgebung zu erkunden, um sich an die sich an verändernde Umweltbedingungen anzupassen. Die räumlichen und zeitlichen Muster von Erkundungsbewegungen sind jedoch kaum verstanden. Wir untersuchten die für Erkundungen typischen Bewegungsmuster adulter Brandgänse (Tadorna tadorna) im Wattenmeer, einem räumlich und zeitlich dynamischen Lebensraum. Dafür wurden neun Brandgänse in angrenzenden Brutgebieten gefangen, mit GPS-Satellitensendern ausgestattet und ihre Bewegungsmuster für 5–18 Monate, hauptsächlich nach der Brutzeit, untersucht. Hierfür verwendeten wir den mittleren täglichen Aufenthaltsort und eine ‚behavioural change point ‘—Analyse. Die einzelnen Brandgänse erkundeten ihre Umgebung an 22% der Tage (Median; Spanne = 6–39%). Meist erkundeten sie für kurze Zeit einen Bereich, in dem sie danach für längere Zeit blieben. Erkundungen waren besonders häufig vor Beginn der mit der Mauser verbundenen Flugunfähigkeit. Brandgänse erkundeten vor Beginn der Mauser das traditionelle Mausergebiet in der Elbmündung intensiv. Die späteren Mauserplätze der einzelnen Vögel wurden jedoch nicht länger oder häufiger als andere Orte in der Elbmündung erkundet. Das Mausergebiet wurde im Median an 59 Tagen genutzt, also etwa doppelt so lange wie die flugunfähige Zeit während der Mauser. Dies deutet darauf hin, dass das Gebiet nicht nur zur Auswahl eines geeigneten Mauserplatzes, sondern auch für andere Zwecke erkundet wurde. Nur zwei Brandgänse erkundeten weit entfernte Gebiete (> 45 km). Wir denken, dass Erkundungsbewegungen es erwachsenen Individuen ermöglichen könnten, sich vornehmlich an kleinräumige Veränderungen der Lebensraumbedingungen anzupassen.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Habitat exploration by birds helps ensure their food supply and safety (e.g., Mettke-Hofmann 2017). Exploratory movements allow individuals to gather information on local habitat quality before deciding where to settle during subsequent days, seasons, or even years. Previous studies reported exploring for molting sites (Fondell et al. 2013; Lewis et al. 2010), breeding sites (Arlt and Pärt 2008; Fijn et al. 2014; Ponchon et al. 2015), and sites for feeding (McEvoy et al. 2015; Vergara et al. 2016). Exploratory movements may also enable birds to adapt to habitat changes, e.g., due to new predators or climate change (McEvoy et al. 2015; Mettke-Hofmann 2017). Exploratory trips may thus have important impacts on individual fitness in terms of survival and reproductive success (Huang et al. 2016; Reed et al. 1999).
Individual frequency and patterns of exploratory movements are poorly understood (Koenig et al. 1996; Ponchon et al. 2013). There are two main reasons for this: First, before the development of small, powerful GPS devices, it was difficult to track the movements of individual birds over a broad geographic and temporal scale. Second, software that could be used to objectively identify the exploration movements has only recently been developed (Gurarie et al. 2016; Kays et al. 2015). Since the improvement in these two methods, studies of exploratory movements have been conducted that revealed some patterns associated with exploratory movements in various bird species: Exploratory movements were shown to be induced by radically changing habitat conditions (McEvoy et al. 2015), failed breeding (Fijn et al. 2014; Ponchon et al. 2017), and were shown to be performed intensively just before periods when individuals were constrained to small ranges, for example before molting of flight feathers including flightlessness (Fondell et al. 2013; Lewis et al. 2010) and breeding (Reed et al. 1999).
Birds living in dynamic habitats in particular would be expected to perform exploration movements (Campioni et al. 2017; Fijn et al. 2014; McEvoy et al. 2015).
One spatially and temporally dynamic habitat for birds is the Wadden Sea. Lunar cycles, wind speed, and direction influence the duration for which a place is flooded, the magnitude and the direction of the current, and thus the composition of the substrate. In addition, winter storms and ice abrasion can affect the distribution of the sediment (Bartholomä et al. 2009). These hydrodynamic factors create a variety of habitats for small macrobenthic animals that live in and shape the mud (Brouwer et al. 2000), and which provide food for millions of birds (Beukema et al. 1993; Horn et al. 2020). Seasonal and annual changes in abiotic environmental conditions, as well as flocks of feeding birds, affect the numbers and body masses of macrobenthic animals, their species composition, and the geographic distribution of larval and adult stages (Beukema et al. 1993; Horn et al. 2020).
In the German–Danish Wadden Sea, we studied the exploratory movements of one bird species: the common shelduck (hereafter Shelducks, Tadorna tadorna). Shelducks in the Wadden Sea feed mostly on small macrobenthic invertebrates by sifting the top layer of the sediment (Anders et al. 2009; Oelke 1979). Their annual cycle includes a complete molt of wing and tail feathers, resulting in a flightless time of 25–31 days between June and September (Hoogerheide and Hoogerheide 1958). During this period, shelducks from most of northwestern Europe have gathered in the wider Elbe estuary (Hoogerheide and Kraak 1942) for at least 200 years (Oelke 1981; Todt 1985). However, the core of the traditional molting area in the Elbe estuary shifted about 20 km southwest about 120 years ago (Glutz von Blotzheim 1988; Goethe 1957), and has since shifted back toward the present core area in recent decades (Kempf and Eskildsen 2000; Todt 1985). Moreover, numbers of molting shelducks in the traditional molting area have recently decreased from about 200,000 (1996–2002) to about 155.000 (2003–2009), corresponding with increased numbers 240 km to the west in the Dutch Wadden Sea from several thousand during the 1990s up to about 60,000 (2010–2012), suggesting that at least some individuals have changed their molting site (Kempf and Kleefstra 2013; Kleefstra et al. 2011). This indicates that shelducks might select the molting site based on ongoing explorations.
This study aimed to identify how, when, and to what geographic extent individual shelducks explored the Wadden Sea during several months in which they were tracked with GPS devices. We identified exploration movements using change point analyses. Specifically, we aimed to: (1) describe how common exploration movements occurred; (2) determine if shelducks explored by roaming movements, visiting many places along the way, or via short-lasting long-distance movements; (3) examine patterns to see if individuals explored places in advance and returned to those places later, or if they decided to stay at the explored places immediately; and (4) investigate if individual molting places were explored with more effort than other areas.
The flightless time during molting is a critical period with potentially strong effects on the fitness. We hypothesized that individual molting sites would be explored with more effort than other areas.
Materials and methods
Study area
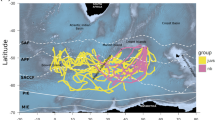
Our study area was the eastern part of the German Wadden Sea (Fig. 1). The area is characterized by tidal flats including tidal channels, sandy shoals, seagrass meadows, sandbars, and estuaries. Shelducks were caught and tagged in nearby breeding grounds (Fig. 1, Table 1).
GPS tagging
We equipped 11 shelducks, aged ≥ 2 years, with 30-g solar-powered GPS devices (Microwave Telemetry, MD, USA) during the breeding seasons 2011 to 2013 (Table 1). Age was determined by the absence of white tips on the secondaries and inner primaries and by pure white wing coverts (Hori 1965), and sex was determined by visual differences in bill and plumage cues (Patterson 1982). We used two types of devices (Table 1): devices using ARGOS satellites to transmit the data (n = 9) were programmed to locate the birds twice a day during the winter (November to February), four times a day during the breeding season (May to June), and hourly otherwise (Fig. 2), and devices using GSM satellites (n = 2) located the birds according to the battery charge, resulting in more frequent location events, sometimes every minute (Table 1). Females (n = 3) were caught at their breeding burrows using a small mist net (mesh size 3 cm) tacked at the entrance. Males (n = 8) were caught using a whoosh net (10 × 5 m, mesh size 4 cm; Pete Reid, UK) combined with a mobile phone-based remote-control release (Kristian Lilje, Germany), placed near burrows where they gathered in groups in the mornings.
Tracking periods for three female and six male shelducks. Three panels are displayed for each individual: the upper panel shows the movement behavior according to BCPA results, the central panel when the individual was located in a possible breeding habitat (usually the catching place) and in the molting area (compare Fig. 1), and the lower panel refers to periods when the individual was incubating or undergoing molting including flightless
GPS devices were attached to the birds as a backpack harness with a 3.8 mm wide Teflon cord (Bally Ribbon Mills, USA) according to Roshier and Asmus (2009). The devices accounted for a mean of 3.1% of body weight for females and 2.5% for males. Birds were measured, weighed, and ringed on their leg with a metal ring (Helgoland bird ringing center) and a coded color ring. One female stopped breeding after catching, the other two bred successfully. Two of the female shelducks were found dead, probably killed by a bird of prey and a fox, respectively, during an unusual cold spell in February 2012 (Schwemmer et al. 2014). Three of the tagged males were observed foraging several weeks after tagging and their behavior and body condition appeared normal. Four of the tagged shelducks were observed after the end of the tracking period and loss of the devices was confirmed (Table 1). We caught more males than females, partly because of the better ratio between the weight of the device and the larger body mass of the males, and partly to avoid females abandon their nest.
Individuals for which we obtained tracking data for more than three months (Table 1) were included in the further analyses. This resulted in the exclusion of two individuals (Table 1).
Classification of behavioral stages
We identified significant changes in the movements of the shelducks using behavioral change point analysis (BCPA; Gurarie et al. 2016). We used the package bcpa version 1.1, implemented in R (R Core Team 2019). BCPA identifies the most likely points in an animal’s track where the movement patterns changed (‘change points’, CPs). The analysis was carried out in two steps: first, persistence velocity (Vp) was calculated for each time step by multiplying the speed of the animal with the cosine of its heading, such that Vp described the displacement of the animal and the tortuosity of its path; second, a user-selected time span (‘window’) moved over estimated values of Vp, its variance, and an autocorrelation parameter, and a CP was set at significant changes of these values. The output file included information on which of the three parameters changed significantly. Daily locations were averaged prior to the analyses because we were interested in broad-scale changes in movement behavior, rather than diurnal patterns (Gurarie et al. 2016). We chose a window size of 40 days to be sufficiently larger than potential changes in the animal’s annual cycle (e.g., incubation time, flightless time during molt; Gurarie 2013; Hoogerheide and Hoogerheide 1958; Patterson 1982). We used the flat output of the analysis and a threshold of 4, i.e., CPs detected by at least four windows were considered (Garstang et al. 2014; Gurarie et al. 2016).
Based on the BCPA results, we assigned movement behaviors to each tracking day: sedentary (≥ 10 days in an area with standardized Vp persistently close to zero), and exploration (standardized Vp moderate, standardized variance high; McEvoy et al. 2015). Exploration thus described a phase during which an individual moved through the landscape, mostly off the mainland, and visited several areas for only a few hours or days, resulting in a high variance of Vp. An example of the assignments can be found in the appendix (Fig. A1 in supplementary material).
The molting period was assigned following Cimiotti et al. (2022): The molting shelducks stayed near the tidal channels during the day at low tide for 24–35 days. During this time, they also did not cross any barriers such as dikes or travel long distances in a short time that would have exceeded the duck’s swimming speed and tidal flow.
Statistical analyses
We tested if different habitat types were explored with more effort. We first classified habitat types (offshore waters, tidal flats, sandbars, foreshore areas, inland wetlands, and inland) using aerial photographs in QGIS (QGIS Development Team 2020). Second, we added the habitat information to all available individual locations. Third, we calculated generalized linear models (GLM) from the package lme4 (Bates et al. 2015). The number of counts per individual in exploratory and the sedentary behavior were included as a dependent variable, and the habitat type was used as exploratory variable. We chose a quasi-binomial error distribution to account for over-dispersion (Korner-Nievergelt et al. 2015; Zuur et al. 2010).
Generalized additive mixed models (GAMMs) were implemented using the package gamm4 (Wood and Scheipl 2016). Circannual patterns were investigated by applying a binomial GAMM with behavior classification per day as a dependent variable and time as an exploratory variable. To display the exploratory effort depending on the flightless period during molt, we centered the time for each individual to the first day of molt. We included time as a smoother using cubic regression splines to account for the nonlinear effect. Individuals were included as the random factor and only time spans for which we had obtained data from at least five individuals were included (Zuur et al. 2014). The number of knots was set to 50 (Ruppert et al. 2003, in Zuur et al. 2014).
We defined the individual molting site by drawing a minimum convex polygon around locations during molt with a buffer of 200-m buffer. This value was chosen because it was about the median distance of successive data points in all GPS-data (172.9 m). Each explored and sedentary area was defined in the same manner.
We determined if the molting area (Fig. 1) was particularly explored in the period before (30 days) compared with the period after molting (30 days) using a GLM. The number of days with exploratory behavior in the molting area was included as a dependent variable, with time as the binary explanatory variable (30 days before vs. after molt). We chose a quasi-binomial error distribution to account for over-dispersion (Korner-Nievergelt et al. 2015; Zuur et al. 2009).
We studied the exploration effort in relation to the distance from sedentary-used areas. For this, we modeled the frequency of exploration in relation to the distance from the edges of sedentary-used areas using a GLMM from the package mgcv (Wood 2011) with a quasi-binomial error distribution to account for overdispersion (Korner-Nievergelt et al. 2015; Zuur et al. 2009). The number of times that an individual was located exploring within a 1-km interval from a sedentary area was used as the dependent variable, and the distance to the closest sedentary area was the exploratory variable. Individuals were included as random factors.
For all the model analyses, we tested violations of the model assumptions visually based on appropriate residual plots (Korner-Nievergelt et al. 2015; Zuur et al. 2009). We tested for over-dispersion by comparing the residual deviance with the residual degrees of freedom (Korner-Nievergelt et al. 2015).
We used non-parametric Kruskal–Wallis tests to test for differences (1) between the distance traveled between sedentary areas, (2) in time spent exploring the molting area compared with other sedentary areas, and (3) in the number of times the molting area was visited compared with other sedentary areas.
Exploratory movements were classified as long-distance exploration movements when they were performed > 45 km from a sedentary area. The value was chosen because it was half of the maximal distance detected between successively used sedentary areas.
Results
Frequency of exploration movements
We detected exploratory behavior on 22% of all days throughout the tracking periods for all nine shelducks (median). The values varied between individuals (range = 6–39%, n = 9; compare Fig. 2). Two shelducks did not molt during the tracking period, presumably due to a lack of time between rising chicks and winter (Cimiotti et al. 2022). Those individuals explored for more days (33% and 39%, respectively) than individuals that did molt (median = 20%, range = 6–30%, n = 7, only data outside of molt included). Differences among individuals were large even when only months for which we had obtained data from all individuals were considered (compare Table 1 and Fig. 2: Jul–Oct; only individuals that molted during the annual cycle were considered; median = 16%, range 6–39%).
Explored habitats
The most frequently visited habitat type was tidal flats (Fig. 3). The sites explored were of the same habitat type as the sedentary sites (Fig. 3). The only difference was found for inland wetlands, which were visited more frequently during sedentary phases (GLM, p = 0.03; Fig. 3).
Spatial scale of exploration movements
Exploration movements were mostly near areas that were also used for sedentary purposes during the study period (Fig. 4; GLMM: R2 = 0.59; deviance explained = 61%, p < 0.001). Almost 25% of locations visited during exploration phases were < 300 m from sedentary locations and about 50% were within 3000 m. Long-distance exploration movements, i.e., ≥ 45 km from sedentary-used areas, accounted for 0.4% of the locations (Fig. 4), and only two shelducks performed such long-distance exploration trips. During one such trip, the individual flew to a salt marsh 63 km north of all sedentary-used areas for 2 days, before returning to the sedentary-used area where it had been throughout the breeding season. The other long-distance exploration movement was performed between molting and winter, when the individual traveled 165 km to the north, left the Wadden Sea, visited the Nissum Fjord, Denmark (56.3°N, 9.2° E), and returned to its previous sedentary area 8 days later. The furthest distance from the sedentary areas explored by most individuals was much closer (median = 23 km, interquartile range (IQR) = 20–31 km). The inter-individual variation was high (goodness of fit, χ2 = 489, df = 8, p < 0.001), with a range of 4–165 km. Map examples of some individual tracks can be found in the online appendix (Fig. A2 in supplementay material).
Exploration effort in relation to distance from sedentary-used areas. Gray dots represent individual proportion explored offset not explored within each kilometer (n = 9 birds). Black line represents the fit from a GLMM with quasi-binomial error distribution and 95% confidence interval (dashed line)
Exploration patterns of sedentary areas
Shelducks explored about half 55% (n = 44) of the sedentary areas. Some of them were visited during exploration periods long after they were used sedentarily, but many of the sedentary areas (48%) were explored before they were occupied. Shelducks usually visited sedentary areas once (median 1, IQR = 1–3 times). On average, the last visit was performed 10 days prior to the sedentary usage (median, IQR = 1–55 days). Each exploratory visit to a subsequently used sedentary area lasted a median of 2 days (IQR = 1–6 days).
When shelducks switched between sedentary areas, they usually did so in an exploratory manner: rather than flying directly to the subsequently used sedentary area, they visited many places along the way, resulting in a tortuous path (examples see appendix, Fig. A2 in supplementay material). It usually took about 7 days to travel from one sedentary area to the next (median, IQR = 3–13 days). The distance was 4.5 times greater than necessary for a direct flight (median, IQR = 2.1–9.6, median distance = 109 km, IQR = 58–240 km vs. a possible direct distance of 28 km, median, IQR = 10–46 km). These patterns did not differ significantly between individuals (Kruskal–Wallis test, temporal: H = 12.6, df = 8, p = 0.13; spatial: H = 12.2, df = 8, p = 0.14).
Exploration of molting sites
The exploratory effort varied throughout the tracking period (GAMM; edf = 10.8; χ2 = 44.95, p < 0.001, n = 7; Fig. 5, only individuals that underwent molt including flightlessness). During the period of 30 days before the start of molting, the probability of exploring was twice as high as during the rest of the tracking period (odds ratio = 2.04, confidence interval = 1.13–3.69). During that time, most locations (median proportion = 1, IQR = 0.74–1) were detected in the molting area (Fig. 2). The molting area was explored more intensively during the 30 days before the onset of molt compared with the 30 days after molt was finished (GLM, p = 0.02; Fig. 6). Combining all locations, shelducks spent a median of 59 days (IQR = 55–67 days) per year in the molting area.
Difference in exploration effort in the molting area 30 days prior to molting compared with 30 days after finishing molting (n = 7). Gray dots: fitted values, black vertical bars: 95% confidence intervals from a GLM with quasi-binomial error distribution (p = 0.02), boxplots: distribution of raw data (Tukey style), EX exploration behavior
Individual molting places were not explored for significantly longer before use (median = 3 days, IQR = 0–10 days) compared with other sedentary areas in the molting area (median = 1 day, IQR = 0–8 days; n = 7; Kruskal–Wallis test, H = 9.37, df = 12, p = 0.67), and were not visited more often (for both: median = 1 time, IQR = 0–3 times; Kruskal–Wallis test, H = 10.6, df = 6, p = 0.10).
Two birds (M3 and M4) were tracked during consecutive molting seasons (Table 1), and both molted at places, 15 and 50 km respectively from the ones used in the previous year. One of the two birds had visited the second molting place five times (9 days) during the previous year. The other bird had visited the second molting place for only about 2 h at the day before the onset of molting, but not at all during the preceding tracking period of > 1 year.
Discussion
Our results showed that shelducks explored extensively, with individual birds using about a quarter of their tracked periods to explore. These findings suggest that birds in dynamic habitats may spend large amounts of time exploring their surroundings.
Our results allow us to interpret the purpose of exploration movements to some extent. Most of the exploration trips in our study were performed by roaming relatively close to intensively used areas, and more-distant exploration movements (> 45 km) were rare. By exploring in small scales, individuals may obtain ongoing information about local environmental changes and habitat cues, such as food, predators, and competing birds (Piper 2011). This information can allow individuals to switch quickly to suitable places if the environmental conditions deteriorate or, in turn, to move to locations with better habitat conditions. We found that sites during exploration phases were mostly located in habitat types that were also used during the sedentary phases. Consequently, tidal flats were most frequently explored compared to other habitat types. This suggests that finding good places to forage may be of high priority.
However, we detected that the probability of exploring was twice as high before molt compared with after molting. This could be explained by several, not mutually exclusive hypotheses. (1) Birds may select a place for the flightless period based on their high vulnerability to predators and downgrading habitat conditions (Dircksen 1968). This hypothesis was supported by our finding that the molting area was explored thoroughly before molt. (2) Alternatively, shelducks may explore intensively before molting to find suitable feeding places for the pre- and post-molting periods. Indeed, we found that shelducks that underwent molting spent a median of 59 days in the molting area, even though the duration of flightlessness was only 25–32 days (Hoogerheide and Hoogerheide 1958). (3) The high levels of exploration coincided with a period when shelducks were expected to feed more, given that hyperphagia and gain of mass before the onset of molt have been detected in numerous geese and duck species (Fondell et al. 2013; Fox et al. 1998; Portugal et al. 2007). Shelducks loose body mass during the molting period (Kempf 1993; Oelke 1969; Patterson 1982), suggesting that they aim to accumulate body reserves before molting.
Long-distance exploration trips involve higher costs compared with small-scale exploration, and the purposes may be different. Long-distance exploration trips may allow individuals to adapt to broad changes in environmental conditions, or to discover better quality areas. Birds can remember specific what/where/when events precisely, sometimes for years (Mayer et al. 2013; Mettke-Hofmann 2017; Roth et al. 2012). For example, adults of some bird species explored for possible breeding sites during or after the breeding period (Arlt and Pärt 2008; Fijn et al. 2014; Ponchon et al. 2015), suggesting that they could remember the explored habitat conditions for longer periods and return later when needed. Our finding that distant areas are explored is consistent with these findings. We suggest that individuals explore distant areas intentionally to enable switching to them. However, we recorded relatively few long-distance exploration movements, suggesting that individuals have limited information to resort to or that they have enough information but their current location is good enough not to leave it.
The exploration effort indicated in our study might have been over- or underestimated due to methodological limitations. Past studies showed that tortuous paths could be associated with exploration movements and foraging movements (e.g., Zhang et al. 2015). To exclude the effect of greater feeding efforts, we assigned the movement phases (exploratory vs. sedentary) based on mean daily locations (Gurarie et al. 2016; see above). However, individuals might leave feeding areas more frequently during periods of high food intake, e.g., because prey density decreases (Anders et al. 2009; Rosa et al. 2008), leading us to overemphasize the amount of exploration. In contrast, the exploration effort might have been underestimated: we obtained geographical data for some of the tracked shelducks at low temporal resolution, e.g., once in 2 h (compare Table 1), possibly leading to missing some short-lasting exploration trips. Individuals briefly visited sedentary-used places in advance, by either leaving their previously used sedentary area and returning to it or switching to a more sedentary behavior in the newly explored area. Other studies found similar results, showing that birds usually used explored habitat conditions shortly before using the areas, i.e., when the information was most valuable and reliable (Lewis et al. 2010; McEvoy et al. 2015; Ponchon et al. 2015). We speculate that shelducks, migrating from the western part of their European range toward the molting area in the Elbe estuary, exhibit similar similar exploratory behavior, and consequently visit many different sites west of the molting area in the Elbe estuary. This hypothesis is supported by Kleefstra et al. (2011), who observed that more shelducks molted in the Dutch Wadden Sea in years with more Sandhoppers (Corophium volutator), a major prey species of shelducks (Buxton and Young 1981).
The selection of sedentary-used areas is likely to be influenced by social contexts, i.e., individuals relying on conspecific social presence (Danchin et al. 2004; Helm et al. 2006). Shelducks are particularly social, e.g., in gregarious courtship groups, feeding flocks during the winter and during the molting period (Patterson 1982). Areas might thus be selected based on a combination of experiences from previous years, contemporary explorations of habitat features, and social information from flocks. The high levels of exploratory behavior found in the current study might thus evolve into collective knowledge of flocks, which might in turn provide reliable information for conspecifics.
We showed that individual shelducks frequently explored the Wadden Sea mostly on small spatial scales and sometimes over long distances. Habitat conditions may change frequently in the Wadden Sea. Exploratory movements of shelducks are likely aimed at finding sites with good habitat conditions for daily needs during different phases of the Shelduck’s annual cycle. Climate change is predicted to have a strong impact on the Wadden Sea and to change habitat conditions for shelducks. Therefore, shelducks may be forced to adapt to these changes, likely involving exploratory movements. Shelducks feed opportunistically on small macrobenthic species that live in the upper layer of the mud. They mainly predate small polychaetes and shellfish < 0.8 cm, but also eat algae, eelgrass, insects, seeds, and diatoms (Anders et al. 2009; Buxton and Young 1981; Evans et al. 1979; Oelke 1979; Thompson 1982; Viain et al. 2011). It is difficult to predict the effects of climate change on the numbers, biomass, and distribution of small macrobenthic species because of different abiotic scenarios (Dissanayake et al. 2012; Hofstede and Stock 2018), as well as the complex ecological interplay among species (Beukema et al. 2009; Buschbaum et al. 2012). The spatial distribution and biomass of small benthic prey species of shelducks is predicted to change in the coming decades. (Beukema et al. 2009; Singer et al. 2016; van Roomen et al. 2012). We speculate that the normal exploratory movements of shelducks will compensate for small-scale geographic shifts and changes in the species composition of small microbenthic animals.
However, predicted sea-level rises suggest that the tidal flats in the Wadden Sea will diminish until the end of the century or will become submerged (Dissanayake et al. 2012; Hofstede and Stock 2018). The rarity of long-distance exploratory movements may not allow shelducks to adapt to such changes. Changing environmental conditions have been shown to induce exploratory movements in other species (McEvoy et al. 2015). It would thus be interesting to see if declining habitat conditions lead to increased exploration by shelducks. We suggest that such information is crucial for estimating the ability of a species to cope with future changes in environmental conditions.
Data availability
Tracking data are archived at Movebank (https://www.movebank.org) and are available on request from the authors.
References
Anders NR, Churchyard T, Hiddink JG (2009) Predation of the shelduck Tadorna tadorna on the mud snail Hydrobia ulvae. Aquat Ecol 43:1193–1199. https://doi.org/10.1007/s10452-008-9216-5
Arlt D, Pärt T (2008) Post-breeding information gathering and breeding territory shifts in northern wheatears. J Anim Ecol 77:211–219. https://doi.org/10.1111/j.1365-2656.2007.01329.x
Bartholomä A, Kubicki A, Badewien TH, Flemming BW (2009) Suspended sediment transport in the German Wadden Sea—seasonal variations and extreme events. Ocean Dyn 59:213–225. https://doi.org/10.1007/s10236-009-0193-6
Bates D, Mächler M, Bolker B, Walker S (2015) Fitting linear mixed-effects models using lme4. J Stat Soft 67:1–48. https://doi.org/10.18637/jss.v067.i01
Beukema JJ, Essink K, Michaelis H, Zwarts L (1993) Year-to-year variability in the biomass of macrobenthic animals on tidal flats of the Wadden Sea: How predictable is this food source for birds? Neth J Sea Res 31:319–330
Beukema JJ, Dekker R, Jansen JM (2009) Some like it cold: populations of the tellinid bivalve Macoma balthica (L.) suffer in various ways from a warming climate. Mar Ecol Prog Ser 384:135–145. https://doi.org/10.3354/meps07952
Buschbaum C, Lackschewitz D, Reise K (2012) Nonnative macrobenthos in the Wadden Sea ecosystem. Ocean Coast Manag 68:89–101. https://doi.org/10.1016/j.ocecoaman.2011.12.011
Buxton NE, Young CM (1981) The food of the Shelduck in north-east Scotland. Bird Study 28:41–48. https://doi.org/10.1080/00063658109476697
Campioni L, Granadeiro JP, Catry P (2017) Albatrosses prospect before choosing a home: Intrinsic and extrinsic sources of variability in visit rates. Anim Behav 128:85–93. https://doi.org/10.1016/j.anbehav.2017.04.008
Cimiotti DS, Hötker H, Garthe S (2022) Unusual pattern of skipped or shortened moulting of flight feathers in late-breeding Common Shelducks. J Ornithol 163:1039–1043. https://doi.org/10.1007/s10336-022-01989-y
Danchin É, Giraldeau L-A, Valone TJ, Wagner RH (2004) Public information: from nosy neighbors to cultural evolution. Science 305:487–491
de Brouwer JFC, Bjelic S, de Deckere EMGT, Stal LJ (2000) Interplay between biology and sedimentology in a mudflat (Biezelingse Ham, Westerschelde, The Netherlands). Cont Shelf Res 20:1159–1177. https://doi.org/10.1016/S0278-4343(00)00017-0
Dircksen J (1968) Brandgans-Mauserzug und tidenbedingte Bewegungen von Brandgans (Tadorna tadorna) und Eiderente (Somateria mollissima) im Raum um Trischen. Vogelwarte 24:179–184
Dissanayake DMPK, Ranasinghe R, Roelvink JA (2012) The morphological response of large tidal inlet/basin systems to relative sea level rise. Clim Change 113:253–276. https://doi.org/10.1007/s10584-012-0402-z
Evans PR, Herdson DM, Knights PJ, Pienkowski MW (1979) Short-term effects of reclamation of part of Seal Sands, Teesmouth, on wintering waders and Shelduck. Oecologia 41:183–206
Fijn RC, Wolf P, Courtens W, Verstraete H, Stienen EWM, Iliszko L, Poot MJM (2014) Post-breeding prospecting trips of adult Sandwich Terns Thalasseus sandvicensis. Bird Study 61:566–571. https://doi.org/10.1080/00063657.2014.950942
Fondell TF, Flint PL, Schmutz JA, Schamber JL, Nicolai CA (2013) Variation in body mass dynamics among sites in Black Brant Branta bernicla nigricans supports adaptivity of mass loss during moult. Ibis 155:593–604
Fox AD, Kahlert J, Walsh AJ, Stroud DA, Mitchell C, Kristiansen JM, Hansen EB (1998) Patterns of body mass change during moult in three different goose populations. Wildfowl 49:45–56
Garstang M, Davis RE, Leggett K, Frauenfeld OW, Greco S, Zipser E, Peterson M (2014) Response of African elephants (Loxodonta africana) to seasonal changes in rainfall. PLoS ONE 9:1–13. https://doi.org/10.1371/journal.pone.0108736
Glutz von Blotzheim UN (1988) Handbuch der Vögel Mitteleuropas, 2nd edn. AULA-Verlag, Wiesbaden
Goethe F (1957) Über den Mauserzug der Brandenten (Tadorna tadorna L.) zum Großen Knechtsand. In: Meise W (ed) Fünfzig Jahre Seevogelschutz. Jordsand Publication, Hamburg, pp 96–106
Gurarie E, Bracis C, Delgado M, Meckley TD, Kojola I, Wagner CM (2016) What is the animal doing? Tools for exploring behavioural structure in animal movements. J Anim Ecol 85:69–84. https://doi.org/10.1111/1365-2656.12379
Gurarie E (2013) Behavioral Change Point Analysis in R: The bcpa package
Helm B, Piersma T, van der Jeugd H (2006) Sociable schedules: interplay between avian seasonal and social behaviour. Anim Behav 72:245–262. https://doi.org/10.1016/j.anbehav.2005.12.007
Hofstede JLA, Stock M (2018) Climate change adaptation in the Schleswig-Holstein sector of the Wadden Sea: an integrated state governmental strategy. J Coast Conserv 22:199–207. https://doi.org/10.1007/s11852-016-0433-0
Hoogerheide C, Hoogerheide J (1958) Slagpenrui van de bergeend, Tadorna tadorna L., in Artis. Ardea 46:149–158
Hoogerheide J, Kraak WK (1942) Voorkomen en trek van de Bergeend, Tadorna tadorna (L.), naar aanleiding van veld-observaties aan de Gooise kust. Ardea 31:1–19
Hori J (1965) Methods of distinguishing first-year and adult Shelducks in the field. Br Birds 58:14–15
Horn S, Schwemmer P, Mercker M, Enners L, Asmus R, Garthe S, Asmus H (2020) Species composition of foraging birds in association with benthic fauna in four intertidal habitats of the Wadden Sea. Estuar Coast Shelf Sci 233:106537. https://doi.org/10.1016/j.ecss.2019.106537
Huang P, Kerman K, Sieving KE, St. Mary CM (2016) Evaluating the novel-environment test for measurement of exploration by bird species. J Ethol 34:45–51. https://doi.org/10.1007/s10164-015-0444-6
Kays R, Crofoot MC, Jetz W, Wikelski M (2015) Terrestrial animal tracking as an eye on life and planet. Science 348:aaa2478. https://doi.org/10.1126/science.aaa2478
Kempf N (1993) Ökologie der mausernden Brandgänse. Jahresber Des Forsch Und Technologiezentrum Westküste 5:103–106
Kempf N, Eskildsen K (2000) Enten im Watt. Landesamt für den Nationalpark Schleswig-Holsteinisches Wattenmeer. Springer, pp 27–30
Kempf N, Kleefstra R (2013) Moulting Shelduck in the Wadden Sea 2010–2012: Evaluation of three years of count and recommendations for future monitoring. Joint Monitoring Group of Migratory Birds in the Wadden Sea (JMMB), Wilhelmshaven
Kleefstra R, Smit C, Kraan C, Aarts G, van Dijk J, de Jong M (2011) Het toegenomen belang van de Nederlandse Waddenzee voor ruiende Bergeenden. Limosa 84:145–154
Koenig WD, van Vuren D, Hooge PN (1996) Detectability, philopatry, and the distribution of dispersal distances in vertebrates. Trends Ecol Evol 11:514–517. https://doi.org/10.1016/S0169-5347(96)20074-6
Korner-Nievergelt F, Roth T, von Felten S, Guélat J, Almasi B, Korner-Nievergelt P (2015) Bayesian data analysis in ecology using linear models with R, Bugs, and Stan. Academic Press Inc., Waltham
Lewis TL, Flint PL, Schmutz JA, Derksen DV (2010) Pre-moult patterns of habitat use and moult site selection by Brent Geese Branta bernicla nigricans: individuals prospect for moult sites. Ibis 152:556–568. https://doi.org/10.1111/j.1474-919X.2010.01023.x
Mayer U, Watanabe S, Bischof H-J (2013) Spatial memory and the avian hippocampus: research in zebra finches. J Physiol 107:2–12. https://doi.org/10.1016/j.jphysparis.2012.05.002
McEvoy JF, Roshier DA, Ribot RFH, Bennett ATD (2015) Proximate cues to phases of movement in a highly dispersive waterfowl. Anas Superciliosa Mov Ecol 3:21. https://doi.org/10.1186/s40462-015-0048-3
Mettke-Hofmann C (2017) Avian movements in a modern world: cognitive challenges. Anim Cogn 20:77–86. https://doi.org/10.1007/s10071-016-1006-1
Oelke H (1969) Körpergewichte von Brandgänsen (Tadorna tadorna) im Mausergebiet Großer Knechtsand (Elbe-/Wesermündung). Vogelkundliche Berichte Aus Niedersachsen 1:47–50
Oelke H (1979) Wovon ernähren sich Brandgänse im Mausergebiet Großer Knechtsand (Elbe-Wester-Mündung). Beitr Zur Naturkunde Niedersachs 32:125–128
Oelke H (1981) Die Brandgans (Tadorna tadorna) in Niedersachsen. Beitr Zur Naturkunde Niedersachs 34:148–156
Patterson IJ (1982) The Shelduck: a study in behavioural ecology. Cambridge University Press, Cambridge
Piper WH (2011) Making habitat selection more “familiar”: a review. Behav Ecol Sociobiol 65:1329–1351. https://doi.org/10.1007/s00265-011-1195-1
Ponchon A, Grémillet D, Doligez B, Chambert T, Tveraa T, González-Solís J, Boulinier T, Rands S (2013) Tracking prospecting movements involved in breeding habitat selection: Insights, pitfalls and perspectives. Methods Ecol Evol 4:143–150. https://doi.org/10.1111/j.2041-210x.2012.00259.x
Ponchon A, Chambert T, Lobato E, Tveraa T, Grémillet D, Boulinier T (2015) Breeding failure induces large scale prospecting movements in the black-legged kittiwake. J Exp Mar Biol Ecol 473:138–145. https://doi.org/10.1016/j.jembe.2015.08.013
Ponchon A, Iliszko L, Grémillet D, Tveraa T, Boulinier T (2017) Intense prospecting movements of failed breeders nesting in an unsuccessful breeding subcolony. Anim Behav 124:183–191. https://doi.org/10.1016/j.anbehav.2016.12.017
Portugal SJ, Green JA, Butler PJ (2007) Annual changes in body mass and resting metabolism in captive barnacle geese (Branta leucopsis): the importance of wing moult. J Exp Biol 210:1391–1397. https://doi.org/10.1242/jeb.004598
QGIS Development Team (2020) QGIS geographic information system. Open Source Geospation Foundation Project. http://qgis.osgeo.org
R Core Team (2019) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna
Reed M, Boulinier T, Danchin E, Oring LW (1999) Informed dispersal: prospecting by birds for breeding sites. Current ornithology. Springer, Cham, pp 189–259
Rosa S, Granadeiro JP, Vinagre C, França S, Cabral HN, Palmeirim JM (2008) Impact of predation on the polychaete Hediste diversicolor in estuarine intertidal flats. Estuar Coast Shelf Sci 78:655–664. https://doi.org/10.1016/j.ecss.2008.02.001
Roshier DA, Asmus MW (2009) Use of satellite telemetry on small-bodied waterfowl in Australia. Mar Freshw Res 60:299–305. https://doi.org/10.1071/MF08152
Roth TC, LaDage LD, Pravosudov VV (2012) Evidence for long-term spatial memory in a parid. Anim Cogn 15:149–154. https://doi.org/10.1007/s10071-011-0440-3
Ruppert D, Wand MP, Carroll RJ (2003) Semiparametric Regression. Cambridge University Press, Cambridge
Schwemmer P, Hälterlein B, Geiter O, Günther K, Corman VM, Garthe S (2014) Weather-related winter mortality of Eurasian Oystercatchers (Haematopus ostralegus) in the northeastern Wadden Sea. Waterbirds 37:319–330
Singer A, Schückel U, Beck M, Bleich O, Brumsack HJ, Freund H, Geimecke C, Lettmann KA, Millat G, Staneva J, Vanselow A, Westphal H, Wolff JO, Wurpts A, Kröncke I (2016) Small-scale benthos distribution modelling in a North Sea tidal basin in response to climatic and environmental changes (1970s–2009). Mar Ecol Prog Ser 551:13–30. https://doi.org/10.3354/meps11756
Thompson DBA (1982) The abundance and distribution of intertidal invertebrates, and an estimation of their selection by Shelduck. Wildfowl 33:151–158
Todt P (1985) Die Mauser der Brandgans (Tadorna tadorna) im Bereich der Insel Trischen. Beitr Zur Naturkunde Niedersachs 38:134–139
van Roomen M, Laursen K, van Turnhout C, van Winden E, Blew J, Eskildsen K, Günther K, Hälterlein B, Kleefstra R, Potel P, Schrader S, Luerssen G, Ens BJ (2012) Signals from the Wadden sea: population declines dominate among waterbirds depending on intertidal mudflats. Ocean Coast Manag 68:79–88. https://doi.org/10.1016/j.ocecoaman.2012.04.004
Vergara PM, Soto GE, Moreira-Arce D, Rodewald AD, Meneses LO, Pérez-Hernández CG (2016) Foraging behaviour in magellanic woodpeckers is consistent with a multi-scale assessment of tree quality. PLoS ONE 11:e0159096. https://doi.org/10.1371/journal.pone.0159096
Viain A, Frédéric C, Delaporte P, Joyeux E, Bocher P (2011) Numbers, diet and feeding methods of Common Shelduck Tadorna tadorna wintering in the estuarine bays of Auguillon and Marennes-Oléron, western France. Wildfowl 61:121–141
Wood SN (2011) Fast stable restricted maximum likelihood and marginal likelihood estimation of semiparametric generalized linear models. J R Stat Soc 73:3–36
Wood S, Scheipl F (2016) Generalized additive mixed models using mgcv and lme4. https://cran.r-project.org/web/packages/gamm4/index.html
Zhang J, O’Reilly KM, Perry GLW, Taylor GA, Dennis TE (2015) Extending the functionality of behavioural change-point analysis with k-means clustering: a case study with the little penguin (Eudyptula minor). PLoS ONE 10:e0122811. https://doi.org/10.1371/journal.pone.0122811
Zuur AF, Ieno EN, Walker NJ, Saveliev AA, Smith GM (2009) Mixed effects models and exstensions in ecology with R. Springer, New York
Zuur AF, Ieno EN, Elphick CS (2010) A protocol for data exploration to avoid common statistical problems. Methods Ecol Evol 1:3–14. https://doi.org/10.1111/j.2041-210X.2009.00001.x
Zuur AF, Saveliev AA, Ieno EN (2014) A beginner’s guide to generalised additive mixed models with R. Highland Statistics Ltd., Newburgh
Acknowledgements
Most of the GPS devices were funded by Naturschutzbund Deutschland e.V. (NABU) via its ‘NABU Club’. The German Federal Environmental Foundation (Deutsche Bundesstiftung Umwelt) supported the research with their Scholarship Programme (AZ 20011/118) and funded one GPS-device. We are grateful to Dominic V. Cimiotti for support in the field and helpful discussions during all stages of the manuscript. Moritz Mercker gave helpful advice on the statistics. Thanks to Hans-Heiner Bergmann, Wolfgang Fiedler, Benedikt Gießing, Klaus Günther, Kristian Lilje, Andreas Schmidt, and Kai-Michael Thomsen for helpful advice and support with fieldwork. We also thank Holger A. Bruns, Brigitte Klinner-Hötker†, and all other fieldwork helpers. Thanks to Katharina Fließbach, Norbert Kempf, Miriam Lerma, Natalie Busch, and Philipp Schwemmer for helpful discussions. Thanks to Susan Furness for language editing.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical statement
All institutional and national guidelines for the equipment and handling of birds were followed. Permissions were given by the Ministry of Energy, Agriculture, the Environment, Nature and Digitalization of the Federal State of Schleswig–Holstein (file number V 312-72241.123-34 (22-2/11)).
Additional information
Communicated by C. Barbraud.
Hermann Hötker was the initiator for this project and sadly died on July 24, 2019. We dedicate this paper to him.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Cimiotti, D.S., Hötker, H. & Garthe, S. Exploratory and seasonal movements of adult common shelducks in the eastern Wadden Sea. J Ornithol 165, 289–300 (2024). https://doi.org/10.1007/s10336-023-02128-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10336-023-02128-x