Abstract
The Agulhas Long-billed Lark Certhilauda brevirostris is restricted to the Agulhas Plain, South Africa, a region extensively transformed for crop production and sheep grazing. We present data on nest and egg characteristics, clutch and brood size, parental care and breeding success previously undescribed for this species. During field surveys in 2020 and 2021, 29 nests were located. Of these, 16 were monitored by camera traps. Laying started in late winter (July) and continued until early summer (late November). Most nests (66%) were in Renosterveld, a unique vegetation component of the Fynbos Biome, with the remainder in human-modified landscapes. Female larks were responsible for nest construction and incubation. Both sexes provisioned nestlings, with provisioning rate related to nestling age and time of day but not brood size. Breeding success was low, with only 14% of nests fledging any young. Only one repeat nesting attempt following a predation event was observed, but the attempt was abandoned. Nest predation was the main cause of nest failure, with eight species of nest predators identified. An apparent preference for nesting in Renosterveld highlights the need for protection of this endangered habitat type. As a ground-nesting species in an agriculturally transformed landscape, this lark faces numerous threats associated with habitat loss, altered predation pressure, exposure to pesticides and disturbance at nest sites.
Zusammenfassung
Die Brutökologie der Agulhas-Langschnabellerche: ein endemischer Vogel, der auf das verbliebene Renosterveld des Westkaps, Südafrika, angewiesen ist.
Die Aghulhas-Langschnabellerche Certhilauda brevirostris ist auf die Agulhas-Ebene in Südafrika beschränkt, eine Region, die extensiv für den Ackerbau und die Schafbeweidung verändert wurde. Wir präsentieren Daten zu Nest- und Eimerkmalen, Gelege- und Brutgröße, elterlichen Pflege und Bruterfolg, die bisher für diese Art nicht beschreiben wurden. Bei den Kartierungen im Feld in den Jahren 2020 und 2021 wurden 29 Nester lokalisiert. Davon wurden 16 Nester mit Kamerafallen überwacht. Das Eierlegen begann im Spätwinter (Juli) und hielt bis in den Frühsommer (Ende November) an. Die meisten Nester (66%) befanden sich im Renosterveld, ein einzigartiger Vegetationstyp des Fynbos, dessen Restbestand in der vom Menschen veränderten Landschaft liegt. Die Weibchen der Langschnabellerche waren für den Nestbau und die Bebrütung der Eier zuständig. Beide Geschlechter versorgten die Nestlinge, wobei die Versorgungsrate mit dem Nestlingsalter und der Tageszeit, nicht jedoch mit der Brutgröße zusammenhing. Der Bruterfolg war gering. Nur 14% der Nester brachten flügge Jungvögel hervor. Nur in einem Fall wurde ein erneuter Brutversuch nach einem Prädationsvorfall beobachtet, der jedoch aufgegeben wurde. Nestprädation war die Hauptursache für den Brutverlust, wobei acht Arten als Nestprädatoren identifiziert werden konnten. Eine offensichtliche Präferenz für das Nisten im Renosterveld unterstreicht die Notwendigkeit des Schutzes dieses gefährdeten Lebensraumtyps. Als bodenbrütende Art in einer agrarwirtschaftlich genutzten Landschaft ist die Aghulhas-Langschnabellerche zahlreichen Bedrohungen ausgesetzt, die mit dem Habitatverlust, dem veränderten Prädationsdruck, der Belastung durch Pestizide und der Störung am Nest einhergehen.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In southern Africa, knowledge of the natural history of some threatened ground-nesting bird species in agricultural regions remains poor, especially with regard to their breeding ecology (Hockey et al. 2005; Maphisa et al. 2009; Engelbrecht and Mashao 2019). Larks, in general, are a poorly known family with 100 species in 21 genera (IOC v13.1). They are largely restricted to the Old World, with the greatest diversity in Africa (de Juana et al. 2004). Almost 30% of the 30–32 lark species found in southern Africa are endemic or near-endemic to the region (Dean and Hockey 1989). Larks are generally associated with low-growing vegetation types in arid to semi-arid environments and agriculturally transformed areas (Dean and Hockey 1989; Winkler et al. 2020). As ground-nesters, larks must mitigate high nest failure rates as well as the impact associated with habitat transformation (e.g., Boyer 1988; Maphisa et al. 2009). One of the endemic South African species that occurs almost entirely in such transformed habitats is the Agulhas Long-billed Lark Certhilauda brevirostris.
The Agulhas Long-billed Lark is part of the Long-billed Lark C. curvirostris sensu lato species complex (Ryan and Bloomer 1999 but see BirdLife International 2016 for differing taxonomic status). In South Africa, the species is listed as Near Threatened due to its small distribution largely outside of formally protected areas (Taylor et al. 2015). Agulhas Long-billed Lark is restricted to the 15,000 km2 Overberg region (Cowling and Heijnis 2001). Virtually unstudied, the breeding ecology of this species is still considered ‘unknown’ (Tarboton 2001; Ryan and Dean 2005). Agulhas Long-billed Lark has limited displacement options as it avoids coastal regions or mountain Fynbos (Evans 2021), preferring the moderately fertile shale-derived lowlands instead, a region that is also home to South Coast Renosterveld (hereafter “Renosterveld”), a unique vegetation type that is highly threatened by extensive transformation into agricultural land (Hoffman 1997; Kemper et al. 2000). Renosterveld is Critically Endangered, with less than 10% remaining in fragments within croplands and pastures (Kemper et al. 2000; Skowno et al. 2019).
Establishing nesting requirements and factors influencing breeding success are important for effective conservation planning (Tapper et al. 1996; Bravo et al. 2020; Ferguson et al. 2021). Parental care strategies vary widely in small terrestrial birds as individuals must balance the need for reproduction with that of survival (Conway and Martin 2000; Burley and Johnson 2002; Cockburn 2006). An understanding of nest placement and parental care behaviour can help predict responses to changing conditions (Conway and Martin 2000; Auer et al. 2007), especially in species in semi-arid to arid regions. Traits such as incubation bout duration can vary with female quality, ambient temperatures or perceived predation risk; with shorter recess bouts minimising cooling and rewarming costs, while longer bouts minimise activity at the nest which reduces predation risk (Williams 1993; Conway and Martin 2000; Visser and Lessells 2001; Deeming 2002; Arct et al. 2022). Provisioning rate can vary with changes in food quality or brood size, nestling age, sex of parent, timing of breeding, as well as ambient temperature and rainfall (Wright et al. 1998; Barba et al. 2009; Engelbrecht and Dikgale 2014; Öberg et al. 2015; Barras et al. 2021; Cauchard et al. 2021; Senécal et al. 2021). Nest location varies too, with ground-nesting species exposed to a unique set of challenges during breeding and face higher predation risk due to the on-ground location of nests (Martin 1993).
We investigated the breeding ecology of Agulhas Long-billed Lark over two nesting seasons in the Renosterveld/agricultural landscape mosaic in the Overberg. We further examined the parental breeding behaviour for this species and documented the roles of male and females, documenting nest attentiveness, duration of incubation bouts, and provisioning rate specifically in terms of variation by an hour of the day, nestling age and brood size. Lastly, we provide details of all predation events that were recorded and estimate breeding success.
Methods
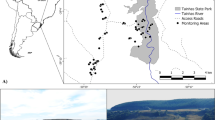
The core study area was located south of Swellendam and east of Protem (34.3° S, 20.3° E) in the southwestern part of South Africa (Fig. 1). The region is characterised by relatively flat lowlands, with the Langeberg Mountains in the north and the Indian Ocean to the south. The area is predominantly an agricultural landscape; patches of different land-use that generally are clearly defined by sharp edges separated by fences or roads. In some instances, natural vegetation (including Renosterveld) is left to grow along fence lines and road verges. A small percentage is urban, utilised for wind energy infrastructure, or protected as nature reserves.
This study took place in regions of Central or Eastern Rûens Shale Renosterveld, depending on the varying geology of the region (Mucina and Rutherford 2006). There are slight differences in rainfall regimes: with Central characterised by winter rainfall (mean annual precipitation 380 mm, range 300–480 mm) while Eastern has both winter and summer rainfall (385 mm, range 270–540 mm). Mean maximum and minimum daily temperatures are nearly similar, Central: 27.3 °C (summer, Jan), 5.6 °C (winter, Jul); Eastern 26.9 °C (summer, Jan), 5.9 °C (winter, Jul) (all data from Mucina and Rutherford 2006). Central Rûens Shale Renosterveld altitude ranges from 20 to 340 m a.s.l., whereas the Eastern Rûens Shale Renosterveld ranges from 40 to 320 m a.s.l. (Mucina and Rutherford 2006). The study area is within the core of the range of the Agulhas Long-billed Lark (https://sabap2.birdmap.africa/species/4123, accessed 25 July 2020, Brooks et al. 2022). In addition to the presence of the study species, the region within the Overberg took into account road access, access to an existing network of landowners, proximity to a wind energy facility and proximity to the largest intact remaining Renosterveld at the Haarwegskloof Renosterveld Reserve (https://overbergrenosterveld.org.za/renosterveld-reserve/).
Nest searches
The study area was visited monthly between August 2020 and January 2022 to determine nesting phenology. To establish where lark pairs or males were present, ‘scouting’ walks were performed from dawn until approximately 11:00 and then from about 14:00 until sunset (although exact start times were temperature dependent). Male Agulhas Long-billed Lark have a distinctive aerial display and call (Ryan and Dean 2005), and both sexes often perch on fence posts, making them conspicuous in the landscape. Once a lark was detected, it was observed for 1–2 h to check for breeding behaviours such as carrying nest material or provisioning food to nestlings. The location of sites where larks or their nests were found was recorded on a hand-held GPS (Garmin 64 series).
Nests were located by targeted searches in areas known to support larks from the scouting walks. When nesting, the females were especially secretive and not easily located. Both the male and female emitted a grating alarm call if an observer approached the nesting area. The level of tolerance varied among adults, with some birds becoming agitated (‘alarming’ and flying around a human intruder) if the observer was within 50 m of the nest, while others only displayed agitation within 10 m of a nest. Most nests were located by carefully checking the area where adults on known territories were flushed. Larks typically flushed when an observer was 60–100 m from the nest, but they had a distinctive flight pattern: ‘shooting’ off the nest, flying fast and low, and landing out of sight. If merely foraging, the birds seemed less concerned with being visible, their flight pattern was slower, with measured wing beats and they flew higher above the ground, often perched within sight about 20–40 m away. After flushing, nesting birds seldom returned within 20 min, therefore observers left the area to avoid disturbance.
Nest locations were assigned to five habitat types: Renosterveld, crop field (oats Avena sativa, barley Hordeum vulgare or wheat Triticum spp., alfalfa Medicago sativa or canola Brassica napus), road verge (narrow strips of vegetation between roads and fences), pasture (used for grazing, no bare soil), fallow pasture (fields that are resting from grazing, with patches of bare soil generally including some shrubs indicative of disturbance). Distance to habitat edge as well as distance to the nearest patch of Renosterveld (in cases where nests were not located in Renosterveld) and patch size were recorded using the Google Hybrid imagery accessed via the open-source software QGIS (2022, http://www.qgis.org/, date accessed: 15 February 2022).
Nest structure, location and type of nest materials used were examined. For nests sheltered by vegetation, the height and type of plant were recorded. For nests found containing eggs, the egg length and width were measured using a small hand-held ruler to the nearest millimetre. Care was taken to minimise contact with nests or nest contents. Nests were not visited when Cape Crow Corvus capensis, Pied Crow C. albus or White-necked Raven C. albicollis were visible in the area, these corvids are known predators of ground-nesting birds that could use visual cues (Troscianko et al. 2016; Ferguson et al. 2021). Egg volume was estimated as egg volume = length × width2 × 0.51 (Hoyt 1979). Besides being useful for comparative purposes, variation in egg volume can have important ecological and evolutionary implications (Boersma and Rebstock 2010). Once nesting activity ceased, the nest depth, internal and external diameter, and orientation of the nest were recorded. The presence of an apron (extended platform on one side of the nest, typically constructed of sticks, thatch and rocks), as well as the size were noted. Apron length was measured from the edge of the nest cup to the end of the apron at the widest point coinciding with the centre point. Five Long-billed Lark nests (assigned to species Certhilauda curvirostris, the super-species pre-split in 1998, Ryan and Bloomer 1999) were recorded in this region during the Southern African Ornithological Society’s Nest Record Card Scheme (NERCS) and these are summarised in the results. Laying dates were either back-dated by comparing the development phase of the nestlings with that of known-age nestlings from this study using photographs or from known hatching dates when nests were found during the egg phase. Assuming an incubation period of 16 days, laying dates for these nests were set at 8 days prior to the date of discovery. Dates were binned into the first or second half of a month to aid interpretation of data. Hatch day was considered day 1.
Nests were visited every 3–5 days until the nestlings fledged or nests were predated. Motion-triggered cameras (Cuddeback 20MP IR—Infrared Flash, H-1453) were positioned 50–80 cm away from nests. Cameras were secured within a small cairn of rocks ~ 30 cm high. The habitat incorporates many small piles of rocks, and the cairns were deemed small enough not to draw specific attention to nest sites. Cameras were not placed at nests within pasture (cameras too conspicuous) or along roads (risk of theft). Once triggered, cameras recorded a photograph followed by a 10 or 30-s video. Cameras reset to renew filming after a period of 5 s (incubation) or 60 s (nestling). Camera settings were altered according to the nesting phase to prolong battery life and minimise the frequency of nest visits: during incubation there were relatively few trigger events with cameras set to record 10-s videos and reset after 5 s. After the eggs hatched, cameras were set to take 30-s videos but only reset after 60 s. Hatch dates were determined from footage of adults removing eggshells and providing food to nestlings.
To test whether there were specific physical habitat characteristics associated with nest sites, we quantified these by adapting the methods used by Erdős et al. (2009). Local habitat characteristics (percentage cover) were investigated within a 25 and 100 cm radius around a nest and compared to a non-nest site within the same territory. Categories recorded were soil, rock, shrubs and grass. Grass and shrubs were included only if their stems or trunks emerged from the ground within the radius. Height (mm) of the tallest bush and grass were recorded at the highest point within the radius. The size (longest dimension) of the largest rocks were recorded (mm). A comparison site was selected by moving 20 m away from the nest site, maintaining the same distance to the edge of the habitat. Comparison sites were chosen to best control for broader habitat-related features so that any local scale differences detected represented true micro-site choice rather than simply representing large landscape-scale differences in vegetation type. Given that nests were often located in relatively small islands of Renosterveld surrounded by agricultural fields, maintaining distance to the edge was important because the centre of the islands were often rocky with dense vegetation.
Behavioural data
The cameras recorded lark behaviour at nests, although large objects (mammals and vehicles) could be detected passing up to 3 m behind nests. All videos were time stamped. The occurrence, start time and duration of the following behaviours were recorded: standing at the nest, provisioning, incubation, faecal sac removal or egg turning.
Male Agulhas Long-billed Lark are larger than females (Ryan and Dean 2005) which made it possible to sex adults in the video footage. For example, measurements taken at the study site showed male mean mass 45.9 g (range = 44.8–47.5 g, n = 3) and culmen 23.4 mm (range = 23.1–23.8 mm, n = 3) versus female mass 35.3 g (range = 32.0–38.3 g, n = 3) and culmen 18.1 mm (range = 17.9–18.5 mm, n = 3) (ATK Lee unpublished data). Based on the time stamps, we calculated the duration of incubation sessions and the provisioning rate (number of provisioning events at a nest per hour).
The reliability of capturing night-time footage was erratic, consequently data used in the behavioural analyses were restricted to daylight hours: between 05:00 and 20:00. Night-time footage was only analysed for the occurrence of predation events (some of which occurred between 05:00 and 20:00). Where predation events were captured on video (n = 11), the identity of the predator as well as the date and time of predation were noted. Causes of failure were not captured in 12 cases because the camera traps were either knocked aside by inquisitive sheep before the nest failed or were not sensitive enough to capture the event.
Statistical analyses
Unless otherwise stated, results are reported as mean values with standard deviation. All statistical modelling was done in Program R (R Development Core Team version 4.1.1 2021). Packages used included tidyverse (Wickham et al. 2019), lme4 (Bates et al. 2015), nlme (Pinheiro et al. 2022), lmerTest (Kuznetsova et al. 2017), and MASS (Venables and Ripley 2002). In all models, residual histograms and scatterplots were visually assessed to confirm model fit.
We used a linear model to test whether there was a relationship between distance to the habitat edge and the patch size of the nesting habitat patch. We used linear mixed models with a normal error structure to test for differences between nest and comparison sites. The response variables were arcsine transformed percentage shrubs, -grass, -soil, -rock, as well as the height of grass, shrubs, and rock size. All variables were checked for normality. As rock size was highly skewed, it was log transformed to improve model fit. The fixed effect was site type, differentiating “nest” and “comparison” sites. Nest ID representing both site types was included as a random effect. Due to sample size constraints, response variables were modelled separately.
We investigated how the duration of incubation sessions varied with the time of day by fitting a linear mixed model with a normal error structure with an hour of the day (both linear and quadratic terms) and date as explanatory variables. The quadratic term was included because a visual inspection suggested a non-linear relationship between these variables. Incubation session duration (measured in minutes) was log transformed to improve model fit. Nest ID was included as a random effect.
We investigated how the provisioning rate (visits per hour) might change with the time of day by fitting a linear mixed model with a normal error structure with an hour of the day (linear and quadratic terms), nestling age, brood size and date as explanatory variables. We also investigated how the faecal sac removal rate (events per hour) might change with the time of day by fitting a linear mixed model with a normal error structure with an hour of the day (linear and quadratic terms), nestling age and brood size as explanatory variables. Including the quadratic term is important as underlying patterns in behaviours are often not accurately captured by a linear term alone but follow unimodal trends (Emms and Verbeek 1991; Conrad and Robertson 1993). Nest ID was included as a random effect.
Daily survival rates were determined using Mayfield’s (1961, 1975) method. The following estimates of breeding success were calculated: the probability of an egg surviving the incubation period (the number of nests lost per number of nest days of incubation) assuming an incubation period of 16 days (n = 1 nest), the probability of a nestling surviving the nestling phase (the number of nests lost per nest-day during the nestling phase) assumed to be 15 days (n = 2 nests), combining the two probabilities to provide a probability of survival of a nest from the start of incubation to the fledgling of nestlings.
Results
We found 29 Agulhas Long-billed Lark nests (3 in 2020 and 26 in 2021). The discrepancy between the two seasons was due to increased insight and knowledge of the species by 2021. Based on the first egg-laying dates (back calculated for 20 nests), egg-laying started in the second week of July (late winter) and continued until early summer (late November) with an egg-phase nest found on 8 December 2021 (Fig. 2). In five instances, active Agulhas Long-billed Lark nests were less than 100 m apart, with the closest being 41 m apart. A further four breeding pairs provided breeding ecology data, two instances involved recent fledglings (still flightless) and two cases in which the nest could not be located but adults were clearly either provisioning young or constructing a nest. There were seven nest record cards for Agulhas Long-billed Lark dating from 1959 to 1988: five containing eggs and two with chicks.
Nest habitat and structure
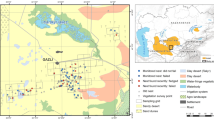
Renosterveld was the most common vegetation type for nest sites (n = 19, 66%), followed by grazed pastures (n = 3, 10%), fallow pasture (n = 2, 7%), grassy road verges (n = 2, 7%), and in croplands of alfalfa Medicago sativa, oats Avena sativa, and a thick stand of alien grass Bromus sp. (each n = 1, 3%) (Fig. 3). The two fledged broods were found in Renosterveld. The mean distance from the edge of the habitat was 29.8 m (± 33.3 m, median = 16.8 m, n = 29). Distance to the edge of the habitat increased with an increase in habitat patch size (F1,24 = 17.6, p < 0.001). For nests in other habitats, the mean distance to the nearest Renosterveld patch was 190.4 m (± 355.3 m, median = 73.1 m, n = 10).
All nests were a shallow cup built on the ground at the base of a small bush (n = 14), planted crop (n = 4) or grass clump (n = 10) (Fig. 4) (Supplementary materials Tab. S1). Typically, at least half of the nest wall was sheltered, resulting in a clear orientation of the nest with a restricted point of access and visibility for the bird on the nest. There was variation in the orientation of the nests: 39% faced southeast, 21% south, 18% northeast, 11% southwest, 7% east, and 4% north. No nests faced west or northwest.
Nest construction behaviour was recorded at four nests, where eggs were laid 10, 7, 5 and 5 days after the nest was first located. In the early construction phase, the nest consists of a messy circle of long grass strands. No construction was observed once the first egg was laid. All 32 clips filmed of nest construction, showed that nest construction was only performed by females. Nests were primarily comprised of coarse dry grass although 31% of nests had tiny pieces of moss incorporated in the nest lining, 28% had twigs supporting the exterior of the nest structure and 17% had dry leaves (from plants other than grasses) (n = 29). Sheep wool was used as nest lining in one nest. One-third of nests had an apron of pebbles or sticks constructed at the nest entrance, which varied considerably in size (Table 1). Nest depth, external and internal nest diameter as well as the apron length and height of sheltering vegetation varied considerably between nests (Table 1).
At a 25 cm radius, there was significantly greater shrub cover (χ2 = 18.81, df = 15, p < 0.001), and less soil cover (χ2 = 8.46, df = 15, p = 0.004) at nest sites versus comparison sites. No significant differences were recorded in the percentage rock (χ2 = 3.51, df = 15, p = 0.06), or grass (χ2 = 3.14, df = 15, p = 0.08) between nest sites versus comparison sites. Shrub height was greater at nest sites (χ2 = 18.33, df = 15, p < 0.001) but there was no difference in grass height (χ2 = 2.10, df = 15, p = 0.15) or rock size (χ2 = 0.10, df = 15, p = 0.76) between the sites. At the 100 cm radius, there were no significant differences in percentages of shrub, soil, or grass (all χ2 < 1.40, df = 15, p > 0.24) or in the height of shrubs or grass or rock size (all χ2 < 1.03, df = 15, p > 0.31).
Description of clutches and eggs
Mean clutch size was 2.7 ± 0.5 (median = 3, range 1–3, n = 20 nests). NERCs showed a mean clutch size of 2.6 ± 0.5 (range 2–3, n = 5 nests) although care should be taken with nest record card data as it is not known whether clutches were complete. The oval-shaped eggs ranged from light creamy brown to a dirty white, flecked with a band of dark brown markings mainly on the broad end of the egg (two examples in Fig. 5). Egg length, width, and volume of 52 eggs varied considerably (Table 2). Within a clutch, length varied by a mean of 1.5 mm (range 0–6 mm), width by 1.1 mm (range 0–2 mm) and volume by 474.1 mm3 (range 0–1754.4 mm3).
Behavioural observations
Sixteen nests contributed behavioural data from camera trap recordings, two during nest construction, 15 during incubation and 10 during the nestling period. Camera traps recorded behaviour at these active nests for an average of 111.1 h (range 2.4–444.7) per nest. The 13 nests lacking behavioural data were because the camera traps malfunctioned (n = 8), or the location of the nests prevented the collection of data (n = 5).
Incubation behaviour was inferred from 767.3 h of camera trapping at 10 nests (range 2.4–197.7 h per nest). In all instances where sex could be determined (98%, n = 289), the incubating bird was female. No evidence of the male feeding the incubating female was observed. The mean and median duration of an incubation bout was 25.1 ± 36.1 and 15.0 ± 36.1 min respectively (range 0.2–278 min, n = 296). The incubation period was 16 days (n = 1) from the last egg laid to hatching. Egg turning was filmed on 24 occasions at seven different nests. It was not possible to ascertain whether incubation started at laying of the first egg or only once the clutch was complete.
Incubation bout duration was significantly explained by an hour of the day and its quadratic term (Table 3). Incubation bouts tended to be longest in the late morning (Supplementary materials Fig S1). The day in the season did not explain variation in the duration of incubation sessions (Table 3).
The altricial nestlings hatched asynchronously with a small amount of white down (Supplementary materials Fig S2). The mean number of nestlings at hatching was 1.9 ± 0.8 (range 1–3, n = 22). During the nestling phase, all adult visits to the nest were assumed to be provisioning events. Prey items were only recorded in 33% of video clips (n = 433). In the remaining clips, the video only started after the adult had entered the nest and presumably had already fed the nestlings. Provisioning rate, faecal sac removal and prey identity were inferred from 1261 nest visits recorded during 637.3 camera trapping hours at 10 nests (range 12.7–165.0 h/nest). Of the suspected provisioning events, 128 were by females and 258 by males, but the sex was unclear in 875 events, precluding any analysis of inter-sex differences in provisioning effort.
Provisioning rate peaked in the late morning (Supplementary materials Fig S3), and increased with nestling age (Supplementary materials Fig S4), but not brood size (Table 3). In 79 (18%) of the recorded provisioning events prey items could be identified. Most prey items were invertebrates, including Lepidoptera (adults 14% and caterpillars 68%), Orthoptera (1%), and Coleoptera (13%). In numerous cases, the items in the bills were tiny, suggestive of grains, small ants or termites.
Two noteworthy interactions with co-occurring ground-nesting species were recorded on camera at nests. In one interaction, a female Agulhas Long-billed Lark removed an African Pipit Anthus cinnamomeus hatchling from its nest. The female lark was at the African Pipit nest. She entered the nest, caught hold of the hatchling by the nape and flew off with it without killing the nestling first. In the second interaction, a female Agulhas Long-billed Lark spent several hours over the course of two days fussing over a Cape Clapper Lark Mirafra apiata nestling. The Agulhas Long-billed Lark appeared to poke the nestling (which was 10 days old) although most of the time it simply stood at the nest entrance preening. The adult Cape Clapper Larks were disturbed by the presence of the larger lark and seemed reluctant to return and feed their nestling. Dense fog prevented clarity on the outcome of this interaction because the nestling disappeared from the nest soon after.
Adults consumed faecal sacs at the nest when nestlings were a few days old but removed sacs from the nesting area once nestlings were older. Faecal sac removal was observed in 146 instances from 9 nests, at a rate of 1.2 ± 0.6 removal events per hour. The rate of removal increased with time of day (Table 3), peaking at midday (Supplementary materials Fig S5) but not with nestling age or brood size (Table 3). Both males (n = 55) and females (n = 29) removed faecal sacs (sex was unknown in 62 cases). In two instances we could establish that the time between hatching and fledging was 12 and 16 days. In a third instance, nestlings were moving around the nest area at 11 days but were still fed in the nest. Nestlings are mobile when they fledge but before their flight or tail feathers are well developed.
Adult birds were frequently filmed (n = 613 video clips) standing at the nest during both the incubation and nestling phases. Standing occurred both before and after incubation and provisioning events. There was no obvious reason for or pattern in the type of behaviour leading to these standing activities although this behaviour could be sentinel behaviour, waiting for nestlings to produce a faecal sac or parents could be providing shade or shelter. Typically, the adult would be positioned ~ 30 cm from the nest, standing on the ground or a rock, scanning the area around the nest. Such standing events were recorded at 14 nests at a rate of 1.7 ± 1.1 events per hour. Mean time spent standing was 0.83 ± 3.50 min. Standing events were recorded during the construction (1.3 ± 0.6 events per hour, n = 3), incubation (1.8 ± 1.2 events per hour, n = 99), nestling (1.8 ± 1.2 events per hour, n = 510 and post fledgling (n = 1) phases. Though both sexes engaged in this behaviour, it involved significantly more females than males (t1234 = − 2.22, p = 0.03, SE = 0.16). Breakdown of the number of events by sex were 238 female, 86 male and 289 unknowns.
Daily survival rate during the incubation phase was 93% (95% CI 89–96%) and during the nestling phase 91% (CI 86–97%). The probability of a nest surviving the incubation phase was 29% (CI 26–32%, n = 18), and of surviving the nestling phase was 26% (CI 21–32%, n = 8). The probability of surviving both phases was 8% (CI 4–11%). The average nestling period was 14 days. Half of the nests that failed were predated (n = 11). Nine nests were predated during incubation. Eight predator species, including mammals (n = 5), reptiles (n = 2) and birds (n = 3) were identified predating Agulhas Long-billed Lark nests (Table 4). All predation events were complete, with all nestlings or eggs consumed. Adult larks attempted to chase snakes from the nest. The two snake predation events solicited strong mobbing responses (spreading wings, mobbing, alarm calling) and a tortoise passing at a separate nest elicited the same response. The 12 nests which failed for unknown reasons probably also were predated as the nests were undamaged. One nest late in the season (21 November 2021) was found to contain two eggs and one nestling was estimated to be 2 days old. The nestling disappeared overnight and the eggs did not hatch. No incidents of nest damage as a direct result of agricultural activities were recorded for the Agulhas Long-billed Lark. For the duration of the fieldwork, recently fledged Agulhas Long-billed Lark was only encountered on five occasions, compared to the many sightings of juvenile Large-billed Galerida magnirostris and Red-capped Calandrella cinerea Larks. One repeat nesting attempt following predation during the egg stage was recorded. The adult birds abandoned the attempt, presumably because the nest predator (Cape Crow) appeared to monitor adult bird behaviour. No repeat broods or replacement broods were recorded. Thorough checks were performed at failed and successful nests to monitor for replacement or repeat nesting behaviour.
Cape Foxes Vulpes chama and Bat-eared Foxes Otocyon megalotis were regularly encountered in the study area, and we expected that they would predate or at least investigate lark nests, but we did not record any footage of foxes near or at Agulhas Long-billed Lark nests. Two Agulhas Long-billed Lark nests were constructed approximately 40 m from active Bat-eared Fox dens. At one site, the Agulhas Long-billed Lark pair was agitated by the presence of the foxes, alarm calling and mobbing the foxes at their den (although the foxes paid no apparent heed to the larks). No interactions were observed between the larks and foxes at the other site. One of these nests was predated by a Yellow Mongoose Cynictis penicillata and the other failed for unknown reasons.
Discussion
This study was the first to establish nesting habitat, timing of nesting, nest characteristics, clutch size, parental roles and nesting success of the range-restricted Agulhas Long-billed Lark. Agulhas Long-billed Lark appear to have nest site preferences on at least three scales: at a landscape scale they prefer Renosterveld habitat; at a patch scale nest placement was farther from patch edges as patch size increased; and at a microsite scale, nests were built in areas of greater vegetation height and cover. Two-thirds of Agulhas Long-billed Lark nests were found in Renosterveld fragments, even though these fragments making up a minor proportion of the greater landscape. Nesting in a fragmented and declining habitat type is likely to have multiple knock-on effects for the species. Agulhas Long-billed Lark face both the direct effects of continued loss of Renosterveld nesting habitat (fewer nesting opportunities) and likely indirect effects resulting from being forced to nest nearer to habitat edges than they might prefer, which can increase predation risk through the edge effect (Tapper et al. 1996; Evans 2004). The small number of nests located in other habitat types (pasture, road verges and alfalfa fields) indicate that Agulhas Long-billed Lark can make use of other habitat types, but nests in these locations are at risk of destruction by farm machinery during planting and harvesting activities.
The nesting season of Agulhas Long-billed Lark is July to December, overlapping with the peak flowering season of Renosterveld (September to October; Curtis-Scott 2020). The nesting season is likely linked to rainfall and might be more protracted farther east where rainfall is more uniform throughout the year. Intense search efforts outside of this period yielded no active nests. Nesting from July to December avoids the temperature extremes of summer as well as overlapping with the abundance of invertebrate food resources during the short flowering season. As ground-nesters, Agulhas Long-billed Lark eggs and nestlings are likely to be negatively affected by both an increase in temperature and the number of rain days if they mis-time nesting, as eggs and nestlings will be exposed to cold and rain (Arlettaz et al. 2010; Öberg et al. 2015).
Like most Alaudidae, the female Agulhas Long-billed Lark was responsible for nest construction and incubation (Winkler et al. 2020), suggesting that the female determines the suitability of nest site and habitat. During construction, the female was often accompanied by the male, i.e., mate guarding. Both adults provisioned nestlings and the provisioning rate increased with nestlings age. It was not possible to determine whether the rate varied between sexes and studies in other lark species have reported mixed results (Engelbrecht 2005; Engelbrecht and Mashao 2019 reported that the rate varied whereas Poulsen 1996, Tieleman et al. 2004; Mwangi et al. 2018 reported not). Parent behaviour at the nest suggested sensitivity to increases in temperature. Incubation bout duration and provisioning rates were highest early in the morning and late afternoon, likely an avoidance of temperature extremes to minimise cooling and rewarming costs (Tieleman et al. 2004; Martin and Camfield 2009). On hot days, female birds were regularly observed shading both nestlings and eggs while panting. Nesting data for the other four Certhilauda long-billed species complex in southern Africa comprises of nest record card (NERCS), unpublished or anecdotal records (Roberts 1940; Keith et al. 1992; Jarvis et al. 1999; Del Hoyo et al. 2004; BD Colahan, WRJ Dean and PG Ryan unpubl. data). As a result, rigorous comparisons within the group are not possible.
The low nesting success recorded for Agulhas Long-billed Lark is not unusual in larks (e.g., Maclean 1970; Engelbrecht and Mathonsi 2012; Engelbrecht and Digkale 2014) but coupled with a preference for a vulnerable and declining nesting habitat might be unsustainable (Oswald and Lee 2021). Habitat loss in agricultural areas is a key driver threatening many bird species (Chamberlain et al. 2000; Donald et al. 2001). We found little evidence of nest defence behaviour by adult larks, except in response to potential reptile predators, despite the numerous instances where larks were observed standing at nests, which suggests that Agulhas Long-billed Lark rely mainly on concealment to avoid nest predation. Like Short-clawed Lark nestlings (Engelbrecht 2005), Agulhas Long-billed Lark nestlings lie very still with no apparent interaction between siblings, nor do nestlings beg audibly when adults arrive with food.
While this study provided valuable insights into the breeding ecology of the Agulhas Long-billed Lark, it is important to acknowledge its limitations. First, only 29 nests were monitored, which limits our ability to draw robust conclusions about some aspects of the species' breeding ecology. Second, the study was conducted in only one part of this highly fragmented landscape, which may not be representative of other areas where the species occurs. Third, while camera traps were used to identify predators, the number of predator species may have been underestimated due to the limitations of the camera trap technology. Snake predation by smaller snakes Rhombic Egg-eater Dasypeltis scabra, may account for the 12 cases in which nest contents disappeared with no obvious indication as to the cause. These smaller snakes do not rear up like the large Boomslang snakes and hence would likely be undetected by the camera traps. Egg-eaters have been recorded as a nest predator at other ground-nesting species (Bates and Little 2013; Oswald et al. 2020) and in a fynbos study site (Nalwanga et al. 2004). We encountered no cases of ‘trampled vegetation’ near nest failures which we did record at several African Pipit, Red-Capped Lark, and Large-billed Lark nests which failed due to agricultural activities (S Rose unpublished data). Finally, the study did not investigate the foraging ecology of the nest predators, this ‘predator point of view’ would provide important insights into the factors driving nest predation rates (Ibáñez-Álamo et al. 2015). Other studies in fragmented landscapes have highlighted how impacts on ground-nesting species vary with predator type (Benson et al. 2010; Krüger et al. 2018; Bravo et al. 2020) but no data exist on predator ecology in the Renosterveld (Topp and Loos 2019).
Despite the limitations of this study, our results do shed light on the breeding ecology and nesting success of the range-restricted Agulhas Long-billed Lark. The findings highlight the likely importance of Renosterveld as a key habitat for this species and the urgent need for conservation measures to protect and restore this habitat. Renosterveld is highly threatened despite being protected and very little exists in formally protected areas which makes it vulnerable to eradication (Curtis-Scott 2020; Moncrieff 2021). Given the high nest failure rates and the diversity of predators observed, there is a need for further investigation into the causes of predation and disturbance. Effective conservation efforts will need to address these factors to improve nesting success and ultimately ensure the long-term survival of this species. Future research should focus on understanding the foraging ecology of nest predators, responses to predation events (renesting), and examine strategies for habitat management and restoration.
Data availability
Not applicable.
References
Arct A, Martyka R, Drobniak SM, Oleś W, Dubiec A, Gustafsson L (2022) Effects of elevated nest box temperature on incubation behaviour and offspring fitness-related traits in the collared flycatcher Ficedula albicollis. J Ornithol 163:263–272. https://doi.org/10.1007/s10336-021-01944-3
Arlettaz R, Schaad M, Reichlin TS, Schaub M (2010) Impact of weather and climate variation on Hoopoe reproductive ecology and population growth. J Ornithol 151:889–899. https://doi.org/10.1007/s10336-010-0527-7
Auer SK, Bassar RD, Martin TE (2007) Biparental incubation in the chestnut-vented tit-babbler Parisoma subcaeruleum: mates devote equal time, but males keep eggs warmer. J Avian Biol 38:278–283. https://doi.org/10.1111/j.2007.0908-8857.04092.x
Barba E, Atienzar F, Marin M, Monros JS, Gil-Delgado JA (2009) Patterns of nestling provisioning by a single-prey loader bird, great tit Parus major. Bird Study 56:187–197. https://doi.org/10.1080/00063650902792049
Barras AG, Niffenegger CA, Candolfi I, Hunziker YA, Arlettaz R (2021) Nestling diet and parental food provisioning in a declining mountain passerine reveal high sensitivity to climate change. J Avian Biol. https://doi.org/10.1111/jav.02649
Bates MF, Little IT (2013) Predation on the eggs of ground-nesting birds by Dasypeltis scabra (Linnaeus, 1758) in the moist highland grasslands of South Africa. Afr J Herpetol 62:125–134. https://doi.org/10.1080/21564574.2013.786760
Bates D, Maechler M, Bolker BM, Walker S (2015) Fitting linear mixed-effects models using lme4. J Stat Softw 67:1–48. https://doi.org/10.18637/jss.v067.i01
Benson TJ, Brown JD, Bednarz JC (2010) Identifying predators clarifies predictors of nest success in a temperate passerine. J Anim Ecol 79:225–234. https://doi.org/10.1111/j.1365-2656.2009.01604.x
Boersma PD, Rebstock GA (2010) Calculating egg volume when shape differs: when are equations appropriate? JFO 81:442–448. https://doi.org/10.1111/j.1557-9263.2010.00300.x
Boyer HJ (1988) Breeding biology of the dune lark. Ostrich 59:30–37. https://doi.org/10.1080/00306525.1988.9633924
Bravo C, Pays O, Sarasa M, Bretagnolle V (2020) Revisiting an old question: which predators eat eggs of ground-nesting birds in farmland landscapes? Sci Total Environ 744:140895. https://doi.org/10.1016/j.scitotenv.2020.140895
Brooks M, Rose S, Altwegg R, Lee AT, Nel H, Ottosson U, Retief E, Reynolds C, Ryan PG, Shema S, Tende T (2022) The African Bird Atlas Project: a description of the project and BirdMap data-collection protocol. Ostrich 93:223–232
Burley NT, Johnson K (2002) The evolution of avian parental care. Philos Trans R Soc 357:241–250. https://doi.org/10.1098/rstb.2001.0923
Cauchard L, Macqueen EI, Lilley R, Bize P, Doligez B (2021) Inter-individual variation in provisioning rate, prey size and number, and links to total prey biomass delivered to nestlings in the Collared Flycatcher (Ficedula albicollis). Avian Res 12:1–10. https://doi.org/10.1186/s40657-021-00247-8
Chamberlain DE, Fuller RJ, Bunce RGH, Duckworth JC, Shrubb M (2000) Changes in the abundance of farmland birds in relation to the timing of agricultural intensification in England and Wales. J Appl Ecol 37:771–788. https://doi.org/10.1046/j.1365-2664.2000.00548.x
Cockburn A (2006) Prevalence of different modes of parental care in birds. Proc R Soc B 273:1375–1383. https://doi.org/10.1098/rspb.2005.3458
Conrad KF, Robertson RJ (1993) Patterns of parental provisioning by eastern phoebes. Condor 95:57–62. https://doi.org/10.2307/1369386
Conway CJ, Martin TE (2000) Evolution of passerine incubation behavior: influence of food, temperature, and nest predation. Evolution 54:670–685. https://doi.org/10.1111/j.0014-3820.2000.tb00068.x
Cowling RM, Heijnis CE (2001) The identification of broad habitat units as biodiversity entities for systematic conservation planning in the Cape Floristic Region. S Afr J Bot 67:15–38. https://doi.org/10.1016/S0254-6299(15)31087-5
Curtis-Scott O (2020) Field guide to Renosterveld of the Overberg. Penguin Random House, Cape Town
de Juana E, Suárez F, Ryan PG (2004) Family Alaudidae (Larks). In: del Hoyo J, Elliott A, Christie D (eds) Handbook of the birds of the world, vol 9. Lynx, Barcelona, pp 496–541
Dean WRJ, Hockey PAR (1989) An ecological perspective of lark (Alaudidae) distribution and diversity in the southwest-arid zone of Africa. Ostrich 60:27–34. https://doi.org/10.1080/00306525.1989.9634502
Deeming C (2002) Avian incubation: behaviour, environment and evolution. Oxford University Press, Oxford
Del Hoyo J, Elliott A, Sargatal J (2004) Handbook of the birds of the world, vol 9. Lynx Edicions, Barcelona
Donald PF, Green RE, Heath MF (2001) Agricultural intensification and the collapse of Europe’s farmland bird populations. Proc R Soc Lond B 268:25–29. https://doi.org/10.1098/rspb.2000.1325
Emms SK, Verbeek N (1991) Brood size, food provisioning and chick growth in the Pigeon Guillemot Cepphus columba. Condor 93:943–951. https://doi.org/10.2307/3247729
Engelbrecht D (2005) Breeding biology of the eastern population of the short-clawed lark in South Africa. Ostrich 76:154–161. https://doi.org/10.2989/00306520509485488
Engelbrecht D, Dikgale L (2014) Breeding ecology of the Chestnut-backed Sparrowlark Eremopterix leucotis in an agroecosystem in the Limpopo province, South Africa. Ostrich 85:67–74. https://doi.org/10.2989/00306525.2014.900829
Engelbrecht DG, Mashao ML (2019) Nesting ecology and parental care of the Sabota Lark (Calendulauda sabota) in a savanna landscape in South Africa. Wilson J Ornithol 131:272–284. https://doi.org/10.1676/18-125
Engelbrecht D, Mathonsi M (2012) Breeding ecology of the Pink-billed Lark, Spizocorys conirostris, in an agricultural landscape in South Africa. Afr Zool 47:26–34. https://doi.org/10.1080/15627020.2012.11407519
Erdős S, Báldi A, Batáry P (2009) Nest-site selection and breeding ecology of Sky larks Alauda arvensis in Hungarian farmland. Ostrich 56:259–263. https://doi.org/10.1080/00063650902791983
Evans KL (2004) The potential for interactions between predation and habitat change to cause population declines of farmland birds. Ibis 146:1–13. https://doi.org/10.1111/j.1474-919X.2004.00231.x
Evans SW (2021) The effects of habitat loss and fragmentation on updated estimates of the population of the Agulhas Long-billed Lark Certhilauda brevirostris, a South African endemic. Ostrich 92:243–256. https://doi.org/10.2989/00306525.2021.1998239
Ferguson AJ, Thomson RL, Nelson-Flower MJ, Flower TP (2021) Conditioned food aversion reduces crow nest predation: an improved framework for CFA trials. J Nat Conserv 60:125970. https://doi.org/10.1016/j.jnc.2021.125970
Hockey PAR, Dean WRJ, Ryan PG (eds) (2005) Roberts birds of southern Africa, 7th edn. Trustees of the John Voelcker Bird Book Fund, Cape Town
Hoffman MT (1997) Human impacts on vegetation. In: Pierce SM, Cowling RM (eds) Vegetation of southern Africa. Cambridge University Press, pp 507–534
Hoyt DF (1979) Practical methods of estimating volume and fresh weight of bird eggs. Auk 96:73–77. https://doi.org/10.1093/auk/96.1.73
Ibáñez-Álamo JD, Magrath RD, Oteyza JC, Chalfoun AD, Haff TM, Schmidt KA, Thomson RL, Martin TE (2015) Nest predation research: recent findings and future perspectives. J Ornithol 156:247–262. https://doi.org/10.1007/s10336-015-1207-4
Jarvis AM, Robertson A, Brown CJ, Simmons RE (1999) Namibian avian database, unpublished. National Biodiversity Programme. Ministry of Environment & Tourism, Windhoek
Keith S, Urban EK, Fry CH (1992) The birds of Africa, vol 4. Academic Press, London
Kemper J, Cowling RM, Richardson DM, Forsyth GG, McKelly DH (2000) Landscape fragmentation in South Coast Renosterveld, South Africa, in relation to rainfall and topography. Austr Ecol 25:179–186. https://doi.org/10.1046/j.1442-9993.2000.01012.x
Krüger H, Väänänen V, Holopainen S, Nummi P (2018) The new faces of nest predation in agricultural landscapes—a wildlife camera survey with artificial nests. Eur J Wildl Res 64:76. https://doi.org/10.1007/s10344-018-1233-7
Kuznetsova A, Brockhoff PB, Christensen RH (2017) lmerTest package: tests in linear mixed effects models. J Stat Softw 82:1–26. https://doi.org/10.18637/jss.v082.i13
Maclean GL (1970) The biology of larks (Alaudidae) of the Kalahari Sandveld. Afr Zool 5:7–39
Maphisa DH, Donald PF, Buchanan GM, Ryan PG (2009) Habitat use, distribution and breeding ecology of the globally threatened Rudd’s lark and Botha’s lark in eastern South Africa. Ostrich 80:19–28. https://doi.org/10.2989/OSTRICH.2009.80.1.3.761
Martin TE (1993) Nest predation and nest sites. Bioscience 43:523–532. https://doi.org/10.2307/1311947
Martin K, Camfield A (2009) The influence of ambient temperature on horned lark incubation behaviour in an alpine environment. Behaviour 146:1615–1633. https://doi.org/10.1163/156853909X463335
Mayfield HF (1961) Nesting success calculated from exposure. Wilson Bull 73:255–261
Mayfield HF (1975) Suggestions for calculating nest success. Wilson Bull 87:456–466
Moncrieff GR (2021) Locating and dating land cover change events in the renosterveld, a critically endangered shrubland ecosystem. Remote Sens 13:834. https://doi.org/10.3390/rs13050834
Mucina L, Rutherford MC (2006) The vegetation of South Africa, Lesotho and Swaziland. South African National Biodiversity Institute, Pretoria
Mwangi M, Njoroge P, Chira R, Gichuki N (2018) Nest food provisioning in the Red-capped Lark Calandrella cinerea does not vary with parental sex differences and time of day. J East Afr Ornithol 38:7–15
Nalwanga D, Lloyd P, du Plessis MA, Martin TE (2004) The influence of nest-site characteristics on the nesting success of the Karoo Prinia (Prinia maculosa). Ostrich 75:269–274. https://doi.org/10.2989/00306520409485454
Öberg M, Arlt D, Pärt T, Laugen AT, Eggers S, Low M (2015) Rainfall during parental care reduces reproductive and survival components of fitness in a passerine bird. Ecol Evol 5:345–356. https://doi.org/10.1002/ece3.1345
Oswald KN, Lee ATK (2021) Population viability analysis for a vulnerable ground-nesting species, the Cape Rockjumper Chaetops frenatus: assessing juvenile mortality as a potential area for conservation management. Ostrich 92:234–238. https://doi.org/10.2989/00306525.2021.1984337
Oswald KN, Diener EF, Diener JP, Cunningham SJ, Smit B, Lee ATK (2020) Increasing temperatures increase the risk of reproductive failure in a near threatened alpine ground-nesting bird, the Cape Rockjumper Chaetops frenatus. Ibis 162:1363–1369. https://doi.org/10.1111/ibi.12846
Pinheiro J, Bates D, DebRoy S, Sarkar D (2022) R Core Team. 2021. nlme: linear and nonlinear mixed effects models. R package version 3.1-153 https://cran.r-project.org/web/packages/nlme/index.html
Poulsen JG (1996) Behaviour and parental care of Skylark Alauda arvensis chicks. Ibis 138:525–531. https://doi.org/10.1111/j.1474-919X.1996.tb08073.x
R Core Team (2021) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. Available: https://www.R-project.org/
Roberts A (1940) The birds of South Africa. Central News Agency, Johannesburg
Ryan PG, Bloomer P (1999) The Long-billed Lark complex: a species mosaic in southwestern Africa. Auk 116:194–208. https://doi.org/10.2307/4089466
Ryan PG, Dean WRJ (2005) Agulhas Long-billed Lark Certhilauda brevirostris. In: Hockey PAR, Dean WRJ, Ryan PG (eds) Roberts birds of southern Africa, 7th edn. Trustees of the John Voelcker Bird Book Fund, Cape Town, pp 880–881
Senécal S, Riva J, O’Connor RS, Hallot F, Nozais C, Vézina F (2021) Poor prey quality is compensated by higher provisioning effort in passerine birds. Sci Rep 11:11182. https://doi.org/10.1038/s41598-021-90658-w
Skowno AJ, Poole CJ, Raimondo DC (2019) National biodiversity assessment 2018: the status of South Africa’s ecosystems and biodiversity; synthesis report. South African National Biodiversity Institute, Pretoria
Tapper SC, Potts G, Royal BMH (1996) The effect of an experimental reduction in predation pressure on the breeding success and population density of grey partridges Perdix perdix. J Appl Ecol 33:965–978. https://doi.org/10.2307/2404678
Tarboton WR (2001) A guide to the nests & eggs of southern African birds. Struik Publishers, Cape Town
Taylor MR, Peacock F, Wanless RM (eds) (2015) The Eskom red data book of birds of South Africa, Lesotho and Swaziland. BirdLife South Africa, Johannesburg
Tieleman BI, Williams JB, Visser GH (2004) Energy and water budgets of larks in a life history perspective: parental effort varies with aridity. Ecology 85:1399–1410. https://doi.org/10.1890/03-0170
Topp EN, Loos J (2019) Local and landscape level variables influence butterfly diversity in critically endangered South African renosterveld. J Insect Conserv 23:225–237. https://doi.org/10.1007/s10841-018-0104-6
Troscianko J, Wilson-Aggarwal J, Spottiswoode CN, Stevens M (2016) Nest covering in plovers: how modifying the visual environment influences egg camouflage. Ecol Evol 6:7536–7545. https://doi.org/10.1002/ece3.2494
Venables WN, Ripley BD (2002) Statistics complements to modern applied statistics with S, 4th edn. Springer, New York
Visser ME, Lessells CM (2001) The costs of egg production and incubation in great tits (Parus major). Proc R Soc Lond B 268:1271–1277. https://doi.org/10.1098/rspb.2001.1661
Wickham H, Averick M, Bryan J, Chang W, McGowan LD et al (2019) Welcome to the tidyverse. J Open Source Softw 4:1686. https://doi.org/10.21105/joss.01686
Williams JB (1993) Energetics of incubation in free-living orange-breasted sunbirds in South Africa. Condor 95:115–126. https://doi.org/10.2307/1369392
Winkler DW, Billerman SM, Lovette IJ (2020) Larks (Alaudidae). Version 1.0. In: Billerman SM, Keeney BK, Rodewald PG, Schulenberg TS (eds) Birds of the world. Cornell Lab of Ornithology, Ithaca. https://doi.org/10.2173/bow.alaudi1.01
Wright J, Both C, Cotton PA, Bryant D (1998) Quality vs quantity: energetic and nutritional trade-offs in parental provisioning strategies. J Anim Ecol 67:620–634. https://doi.org/10.1046/j.1365-2656.1998.00221.x
Acknowledgements
Funding for this study was provided by BTE Renewables (formerly BioTherm Energy) and the South African National Research Foundation. Field observations were assisted by Barbara le Roux, Felicity Ellmore, Ross Soller, Toni Hoenders and Vince Ward. This research would not have been possible without access to farms provided by Hermi Steyn, Gawie Gilliomee, Johan Albertyn, Johnico Swart, Santie Steyn and Piet Steyn; Martie Gilliomee kindly provided accommodation. Dr Odette Curtis-Scott and Grant Forbes from Overberg Research Conservation Trust provided invaluable field-based support, including accommodation on the Haarwegskloof Renosterveld Reserve.
Funding
Open access funding provided by University of Cape Town.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Ethical approval
Ethical clearance for this study was granted by the University of Cape Town’s Science Animal Ethics Committee under application number 020/V19/RT.
Additional information
Communicated by C.T. Downs.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Rose, S., Thomson, R.L., Lee, A.T.K. et al. The breeding ecology of the Agulhas Long-billed Lark: an endemic bird dependent on the remnant Renosterveld of the Western Cape Province, South Africa. J Ornithol 165, 391–404 (2024). https://doi.org/10.1007/s10336-023-02123-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10336-023-02123-2