Abstract
Urbanization, industrialization, and intensification of agriculture have led to considerable heavy metal pollution across the globe, harming our ecosystems. Concentrations of arsenic (As), cadmium (Cd), copper (Cu), and lead (Pb) have been analysed in 249 eggshells collected between 2006 and 2021 from 83 female Common Cranes (Grus grus) nesting within north-eastern Germany. Information on the presence of trace elements in cranes from Europe and their potential adverse effects on the reproduction are largely missing. Only Cu and Pb were found to be present in eggshell samples. Levels of both metals did not exceed concentrations considered potentially toxic in birds and unhatched eggs did not contain higher metal concentrations compared to eggshell residues from hatched eggs. Statistical analysis revealed that trace element concentrations decreased significantly over the course of the study period. The ban of leaded gasoline in the early twenty-first century and strict limitations of heavy metal-based biocontrol products are likely responsible for this decrease over the years. However, as Cu levels gradually increase with increasing proportions of agricultural areas within the cranes’ home ranges, we suggest that considerable amounts of Cu originating from agricultural practises are still being released into the environment. We found no increase in metal concentrations in eggshells with increasing female age, suggesting that heavy metals do not accumulate in the circulatory systems of the adults over time. This study is the first to assess heavy metal contamination in Common Cranes and indicates the suitability of crane’s eggshells as bioindicator for monitoring environmental pollution.
Zusammenfassung
Schwermetallrückstände in Eischalen Eurasischer Kraniche ( Grus grus) aus einer landwirtschaftlich geprägten Region Nordostdeutschlands.
Urbanisierung, Industrialisierung und die Intensivierung der Landwirtschaft haben weltweit maßgeblich zur Belastung und Schädigung unserer Ökosysteme durch Schwermetallrückstände beigetragen. In 249 Eischalenresten von 83 verschiedenen Weibchen des Eurasischen Kranichs (Grus grus) aus dem Zeitraum von 2006 bis 2021 wurden Rückstände von Arsen (As), Cadmium (Cd), Kupfer (Cu) und Blei (Pb) analysiert. Informationen bezüglich einer möglichen Kontamination der Art in Europa mit den untersuchten Schwermetallen sowie deren mögliche negative Auswirkungen auf den Reproduktionserfolg fehlen bisher. Im Rahmen der Analysen wurden in den Eischalen nur Rückstände der beiden Elemente Cu und Pb festgestellt. Gemessene Konzentrationen lagen jedoch unter jenen, welche für Vögel als potenziell toxisch gelten. Eischalen fauler Eier enthielten keine signifikant höheren Metallkonzentrationen verglichen mit Eischalenresten geschlüpfter Eier. Die statistische Auswertung zeigte, dass die Konzentrationen der nachgewiesenen Spurenelemente im Lauf des Untersuchungszeitraumes signifikant abgenommen haben. Ursächlich hierfür könnte, neben dem Verbot bleihaltigen Benzins im frühen 21. Jahrhundert, die generelle Limitierung von auf Schwermetallen basierenden Pestiziden sein. Allerdings weisen steigende Cu-Konzentrationen mit zunehmendem Anteil landwirtschaftlich genutzter Flächen im Aktionsraum eines Kranichpaares darauf hin, dass noch immer nicht zu vernachlässigende Kupfereinträge in die Umwelt im Rahmen der landwirtschaftlichen Praxis stattfinden. Hinweise, dass die Konzentrationen von Schwermetallen in Eischalen mit zunehmendem Alter der Weibchen steigen, wurden nicht gefunden. Dies legt nahe, dass Schwermetalle wie Cu und Pb mit der Zeit nicht im Kreislaufsystem adulter Tiere akkumulieren. Diese Studie befasste sich als erste mit der möglichen physiologischen Belastung Eurasischer Kraniche durch potenziell toxische Schwermetallrückstände und zeigt die Eignung von Kranicheischalen als Bioindikatoren für Umweltveränderungen.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Increasing industrialisation, mining activities, and intensive agriculture cause environmental pollution putting our ecosystems and biodiversity at risk (Rajput et al. 2017; Morin-Crini et al. 2022). Toxic heavy metals form a significant proportion of pollutants contaminating our environment (Tumanyan et al. 2020). Pollutants are directly released into the environment by applying fertilizers or pesticides in agriculture or via waste waters and gas emissions (Fuchs et al. 2010; Kraus and Römer 2019). In addition, traffic represents a major source of emission of lead (Pb) and copper (Cu). Despite the reduction of lead additives for gasoline in Europe starting in the 1980s, leaded gasoline continued to be used by several European countries until 2002 and material abrasion still causes Pb and Cu to be released into the environment (Penkała et al. 2018). Heavy metals such as mercury (Hg), selenium (Se), cadmium (Cd) or arsenic (As) are mainly emitted during industrial processes (German Environment Agency 2023). However, traces of Cu, Cd, Pb, and Se are also found in pesticides, fertilizers or food supplements for livestock farming (e.g., Sager 2006; Brandt et al. 2010; Kumar and Yaashikaa 2019). Environmental contamination, with non-essential elements in particular, is higher within areas showing high human activity (Ruuskanen et al. 2014; Chen et al. 2016). Whereas highest environmental contamination with Cd is associated with waste and sewage disposal facilities, Cu and Pb levels are considerably elevated in regions dominated by intensive agriculture (Ruuskanen et al. 2014; Chen et al. 2016).
These substances accumulate in the environment over time, and being inhaled, ingested, or taken up by plants consequently accumulate in the food web (e.g., Franson and Pain 2011; Wayland and Scheuhammer 2011; Tumanyan et al. 2020). Facing climate change, the effects of environmental conditions in terms of temperature changes or changes of water availability on the heavy metal uptake by plants and animals should be considered. Whereas few studies on element availability in the environment in relation to abiotic and geochemical parameters exist (e.g., Urbina et al. 2015; Costa et al. 2020), studies focusing on differences in heavy metal presence in organisms under changing environmental conditions are missing. High levels of non-essential metal elements were found to cause physiological dysfunction or reduced reproductive success and survival in different bird species (e.g., Spahn and Sherry 1999; Zhang and Ma 2011; Almalki et al. 2019). Extensive knowledge of a species biology and ecology are essential to analyse and truly understand the effects of trace elements on an organism (Furness 1993). Especially Pb and Cd, but also Cu and Se, have been linked to reduced reproductive performances in birds (Spallholz and Hoffman 2002; Kertész et al. 2006) and changes in egg morphology have been related to the presence of heavy metals (e.g., Gonzales and Hiraldo 1988). However, the minority of studies investigated contaminant exposure and variables affecting heavy metal levels in free-ranging birds (Brown et al. 2018).
Top-level predators and long-living species are considered particularly vulnerable to negative effects of heavy metals (Rattner 2009). However, studies on the bioaccumulation of trace elements in tissues of birds over time are largely missing, even though Gochfeld et al. (1996) found that metal concentrations in different tissues of Laughing Gulls (Larus atricilla) increased with increasing age. The most common heavy metal contamination source in raptors is lead poisoning through the ingestion of leaded ammunition. Less specialised omnivorous species such as cranes are not only exposed to leaded ammunition as well (e.g., Teraoka et al. 2007), but experience heavy metal contamination through accumulation of trace elements from various sources of environmental pollution (Monclús et al. 2020). Agricultural activities were found to be linked to increases in heavy metal concentrations in ditch and riparian wetlands in China (Jiao et al. 2014). Therefore, cranes nesting within habitats in agricultural fields could be exposed to higher doses of trace elements compared to individuals nesting within more natural habitats, e.g., within forested areas. Considering this, and because of their territoriality and high nesting site fidelity (Mewes 2017), cranes are particularly suitable to be exposed to trace elements and its effects within certain areas.
Within the family Gruidae studies on heavy metal contamination mainly focused on endangered species, such as Red-crowned Cranes (Grus japonensis; Luo et al. 2013 [feathers, feces]; Luo et al. 2016 [eggshells]) or Whooping Cranes (Grus americana; Lewis et al. 1992 [soft tissues and egg content]). Information on the status and the effects of trace elements on less endangered crane species, especially from Europe, are lacking. Due to increasing population numbers, Common Cranes (Grus grus) have shown high flexibility in the use of nesting habitats. Whereas Common Cranes were formerly known to prefer forested areas and rather remote mire complexes for nesting, part of the German population displayed a shift from wooded habitats into the open landscape (Mewes 2010). Since 1996, the proportion of cranes nesting within agricultural landscapes has increased from 8.0% to more than 30.0%. According to numerous studies linking heavy metal pollution to agricultural activities (e.g., Kumar and Yaashikaa 2019), it has to be considered that cranes populating intensively used agricultural areas might suffer from elevated levels of heavy metal contamination, causing physiological stress. To assess the status of heavy metal contamination of Common Cranes in north-eastern Germany, we conducted heavy metal analyses of eggshell residues. The crane breeding population within our study area has been monitored extensively for more than 30 years, including the collection of eggshell residues and recording of the reproductive history of single females for up to 30 years.
Using avian eggshells and feathers for trace element analyses has been gaining great attention over the last decades. Collecting eggshell fragments or moulted feathers represents a non-invasive method enabling large sample sizes from free ranging individuals. Despite potential effects of heavy metals on the reproductive success of a species, individual birds, their eggshells, and feathers may also function as biomonitors of environmental pollution (Zhang and Ma 2011; Ashkoo et al. 2020; Lin et al. 2021). Eggshell and feather formation are known to function as excretion systems for environmental contaminants in birds, such as heavy metals (Burger 1994; Martínez et al. 2012). Our study is the first to assess heavy metal content in eggshells of Common Cranes. To gain a wide overview on the effect of trace elements in cranes in northern Germany we included individual-based variables, e.g., nesting habitat or age, as well as environmental variables, e.g., landscape composition and weather parameters, in our analyses.
The main objectives of our study were (1) to assess changes in heavy metal content of eggshells over the last 15 years (As, Cd, Cu, Pb); (2) to determine whether heavy metal concentrations are higher in eggshells from nesting habitats surrounded by intensively used agricultural fields; (3) to determine whether metal concentrations vary according to the landscape composition of cranes´ home ranges; (4) to analyse if metal concentrations in eggshells increase with female age; (5) to determine if eggshells of unhatched eggs show higher metal concentrations, and (6) to investigate the effects of weather parameters on metal concentrations in eggshells.
Materials and methods
Study area
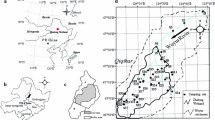
The study area Goldberg is part of the German Northern Lowland and comprises a region in the southern part of the German state of Mecklenburg–Western Pomerania extending from the Plauer See in the East (53° 29′ 27.8″ N 12° 16′ 51.9″ E) to the city of Parchim in the West (53° 25′ 45.9″ N 11° 50′ 45.7″ E, Fig. 1A). The study area consists of agricultural fields (66.3%), scattered woodland areas (13.8%), and grasslands (11.0%). The remaining 8.9% comprise a variety of water bodies, such as rivers, lakes, swamps, and different kinds of pothole wetlands (Mewes 2014). Annual precipitation ranges from 591.0 to 639.2 mm and annual average temperature from 8.8 to 9.0 °C (Waren [Müritz]/Schwerin, German Meteorological Service DWD 2018).
A Distribution of sampling sites across the study area ‘Goldberg’, including defined home ranges. B Example of one nesting site and defined home range, including the latest mapping of biotopes and land use parameters, on which the analyses of landscape compositions are based on (LUNG 2010)
Sample collection
Collection of eggshell remains was part of an extensive breeding site monitoring and each year took place several days after juveniles had hatched and crane families were not present at the nesting sites to avoid disturbance. Since only small eggshell pieces were collected from the nests it was not possible to determine from which region of the egg the fragments came from. In addition, unhatched and addled eggs (n = 35), hereafter referred to as addled eggs in general, were collected as well, after having ensured that no chick will hatch. This was the case when either chicks did not hatch after 35–40 days of incubation or the egg had a foul smell. After collecting the samples, addled eggs were opened and the egg content was removed. Obvious dirt particles as well as inner shell membranes were removed from eggshells using deionised water. Eggshell remains were fully dried and stored under dry conditions. 249 samples, collected between 2006 and 2021, were selected to be included in the heavy metal analyses.
Element analysis
Dry eggshell material (0.5 g) was dissolved in high-purity nitric acid (1.0 ml HNO3, ROTIPURAN®, 65%) and incubated for 24 h at 110 °C. Afterwards, dissolved eggshells were diluted 1:10 with high-purity water. Graphite Furnace Atomic Absorption Spectrometry (GF-AAS, Analytik Jena contra700, Jena, Germany) was used to determine heavy metal contents. Elements analysed included As, Cd, Cu, and Pb. Low, medium, and high concentrations, exact concentrations and range depending on the limit of quantification of each element, were chosen for the calibration curves. The measurement process was validated by determining recovery rates from the biological matrix using a spiking technique as described by Ullah et al. (2017). Defined amounts of each element were added to eggshell material before processing. Heavy metal concentrations of spiked samples were measured and recovery rates determined. Average recovery rates ranged from 97 to 102%. Limits of detection (LOD) have been calculated for each trace element according to the formula \(\mathrm{LOD}=\frac{3.3\upsigma }{\mathrm{S}}\) (σ = standard deviation of response; S = slope of the calibration curve; ICH guideline Q2 [R2] 2022). LOD for As, Cd, Cu, and Pb were 7.1 µg L−1 (0.007 ppm), 2.8 µg L−1 (0.002 ppm), 4.4 µg L−1 (0.004 ppm), and 1.1 µg L−1 (0.001 ppm), respectively. All samples were measured in batches that included blanks and a standard calibration curve. Three replicates of each sample were measured for process validation. In case relative standard deviations (RSDs) exceeded 10%, single outliers of extinction values were removed or the measurement was repeated. Element concentrations were reported in parts per million (ppm) of dry weight (dw).
Additional parameters
Additional data collected in the field included habitat parameters and exact nest locations. Habitats were categorized according to their position in the landscape (‘woodland’, ‘open landscape’ or ‘intermediate habitats’ located in between), hereafter referred to as habitat location (HLocation), as well as habitat type (HType) and dominant vegetation (DVegetation). Nesting sites could be assigned to eight different habitat types, including ‘abandoned meanders’, ‘silted ponds’, ‘marshy meadows’, ‘mire complexes’, ‘pothole wetlands on farmland’, ‘pothole wetlands on grassland’, ‘forest potholes’, and ‘swamp forests’. Vegetation types included habitats dominated by either Alnus glutinosa, Carex spec., Juncus spec., Phragmites australis, Typha spec., Salix cinerea, mixed vegetation, or others. Nest locations were assigned a position along a longitudinal west–east (WE-gradient) and latitudinal south–north gradient (SN-gradient) to analyse effects of a gradually changing landscape composition. To analyse the effect of landscape composition we defined an average home range of two kilometers around each nesting site (Fig. 1A, B). Using QGIS (Version 3.4.15) evenly distributed points (every 50 m) were created for each action scope. The latest mapping of biotopes and land use parameters provided by the’Landesamt für Umwelt, Naturschutz und Geologie’ (LUNG) was used to extract biotope information for each point. Generated data were used to calculate proportional landscape composition of each action scope surrounding a nesting site (Fig. 1B). Identified biotopes were summarized into the following categories: Agriculture (includes crop, fruit and vegetable cultivation), Infrastructure (includes streets, railroads, settlements, single buildings, economic facilities, hydro-engineering installations, supply and disposal facilities, agricultural storage areas etc.), Water (lakes, rivers, streams, mires, pothole wetlands, ditches, temporary and permanent smaller water bodies etc.), Grassland (grasslands, wet grasslands, dry grasslands, pastures) and Forest (mixed forests, coniferous forests, deciduous forests, copses, hedges, single trees, groves).
Weather parameters were provided by the German Meteorological Service (DWD, Deutscher Wetterdienst). Variables considered included the Coldsum of the preceding winter—a measurement to account for the harshness of a winter, the total amount of Precipitation prior to the breeding season (November–April in millimeters) and the total amount of Sunshine duration during the breeding season (March–May in hours).
Applying methods established by Mewes and Rauch (2010), Schmitz Ornés et al. (2014), and Höltje et al. (2016) we were able to assign clutches to specific females based on morphological features of the eggs. On average, Common Cranes start reproduction at the age of 4 years (Prange et al. 2016). In addition to that, constant comprehensive nesting site monitoring and high nesting site fidelity (Mewes 2017) enabled age estimations and the recording of reproductive history of single female cranes for up to 30 years. Because most individuals are not marked, age can not be known exactly, but females appearing for the first time were estimated to be 4 years at that time.
Statistical analyses
Statistical analyses were performed using R (R Core Team, Version 4.1.3) and RStudio (Version 2022.02.0) assigning significance at p values ≤ 0.05. Packages used included ‘lme4’ (Bates et al. 2015), ‘car’ (Fox and Weisberg 2019) and ‘performance’ (Lüdecke et al. 2021). We applied generalized linear mixed models (GLMM) for each element separately, specifying a gamma error distribution and log link function to analyse effects of habitat parameters, weather parameters, and individual-based parameters on heavy metal concentrations of eggshells. Since the data set included repeated measures from the same individuals the variable Female was included as random factor. Following Martín-Fernández et al. (2003) zero-values were replaced with 65% of the detection limit, since proportions of measurements below LOD were fairly low (< 5%). Models were built step by step, eliminating non-significant variables to avoid collinearities. Model selection was based on the Akaike’ information criterion (AIC). Spearman's rank correlation was applied to test for relationships between landscape composition and longitudinal or latitudinal gradients, respectively. Due to collinearity issues and small sample sizes for single habitat types, the variable HType was not included in final models. The same holds for the variable Forest which is part of the biotopes used for the assessment of landscape composition.
Results
Out of 249 egg shell samples almost all contained Cu and Pb but neither As nor Cd could be detected in any of the samples analysed (Fig. 2). Therefore, no statistical analyses were applied for the elements As and Cd.
Parameter correlations
The amount of Cu and Pb in eggshells was found to be weakly correlated (Table 1). Results of correlation analyses of landscape compositions and longitudinal and latitudinal gradients, respectively, suggested an increasing presence of waterbodies along the WE-gradient (Table 1) and a decreasing presence of agricultural areas from south to north (Table 1). Other landscape components were not or only weakly correlated with the longitudinal and latitudinal gradients. Based on this, we assumed landscape composition to change along the SN-gradient from higher human impact (greater proportion of agriculture) in the south towards more natural areas with less human impact in the northern part of the study area.
Copper
On average, eggshells of Common Cranes from our study area contained 0.910 ppm copper, ranging from below detection limit (ND) to 6.976 ppm. In eight samples no copper could be detected. Concentrations of Cu decreased significantly over the years (Fig. 3; GLMM, Est. = − 0.105, SE = 8.75e−05, p < 0.001). Highest element levels were detected in samples from 2008 (6.98 ppm) and 2009 (5.43 ppm; Fig. 3A). Individual-based factors Age and Clutch were found to have no effect on Cu concentrations (Table 2) and no difference was found between eggshells from unhatched addled and post-hatched eggs (Fig. 3B, Table 2). Weakly significant effects of Dominant vegetation are negligibly. Concentrations seem to be higher in eggshells from nesting sites dominated by Juncus spec. (GLMM, Est. = − 1.286, SE = 0.588, p = 0.029), but the number of corresponding nesting sites is very low (n = 2), and therefore, results are not considered reliable. Single parameters of landscape composition had no effect on Cu concentrations but concentrations of Cu change along the SN-gradient. Concentrations are lower in samples from nesting sites located farther north (GLMM, Est. = − 2.3e−03, SE = 1.02e−03, p = 0.027). Concerning weather parameters only the harshness of the preceding winter was found to affect concentrations of Cu in the eggshells (Fig. 4). Element concentrations were found to be particularly low after strong winters (Fig. 4; GLMM, Est. = − 3.3e−03, SE = 6.68e−04, p < 0.001).
Lead
On average, eggshells of Common Cranes from our study area contained 0.092 ppm lead, ranging from ND to 0.755 ppm. Twelve samples did not contain lead concentrations above the detection limit. Concentrations of Pb decreased significantly over the years (GLMM, Est. = − 0.015, SE = 3.82e−04, p < 0.001). However, the highest concentration of Pb was detected in a sample from 2015 (0.76 ppm; Fig. 5A). Surprisingly, levels of Pb in eggshells were found to decrease with increasing female age (Est. = − 5.51e−02, SE = 2.61e−02, p = 0.035). Individual-based factor Clutch did not affect levels of Pb (Table 2) and no elevated concentrations of Pb were found within samples from unhatched addled eggs (Fig. 5B, Table 2). Dominant Vegetation showed no distinct effect on levels of Pb (Table 2) but tend to be lowest in egg shells from habitats dominated by Typha spec. (0.046 ppm, n = 15; Fig. 5C), whereas highest mean concentrations were found in egg shells from habitats dominated by Salix cinerea (0.174 ppm, n = 14; Fig. 5C). No changes in concentrations of Pb were detected along longitudinal or latitudinal gradients. Concomitantly, single landscape parameters were not found to significantly affect Pb levels (Table 2), but concentrations tend to slightly increase with increasing proportions of infrastructural components within the defined home ranges of cranes (Est. = 0.173, SE = 9.91e−02, p = 0.080). Concerning weather parameters, precipitation prior to the breeding season had an effect on concentrations of Pb in eggshells (Fig. 6A). Concentrations decreased with increasing precipitation (Fig. 6A; GLMM, Est. = − 7.84e−03, SE = 2.75e−03, p < 0.001). Other weather parameters did not affect Pb levels.
Discussion
This is the first study to investigate heavy metal residues in eggshells of Common Cranes and, in general cranes within Europe, assessing long-term changes in heavy metal content of eggshells from free living cranes over the course of 16 years.
Copper and lead
The majority of samples contained Cu and Pb, although concentrations found are not considered to be potentially toxic (Franson and Pain 2011). However, single eggshells contained concentrations of Pb markedly exceeding suggested background Pb levels in wild birds (< 0.2 ppm dry weight; Franson and Pain 2011), indicating increased heavy metal intake within our study area. Heavy metal concentrations in eggshells represent recent heavy metal intake of egg-laying females (Franson and Pain 2011), which also explains the similarity of metal levels found in first and replacement clutches of single individuals. Concentrations of Pb found are similar to those reported for Red-crowned Cranes (Grus japonensis; Teraoka et al. 2007) but it is important to take into account that Teraoka et al. analysed metal levels in soft tissues. In general, heavy metal concentrations are significantly lower in eggs compared to soft tissues of adult birds, but concentrations have been shown to be correlated (Jeng et al. 1997; Luo et al. 2016). This implies that only a limited proportion of heavy metals is being eliminated from the adult female during egg formation, suggesting that the heavy metal load of adults could be several times higher. Anyway, the presence of non-essential elements above background levels in eggshells can be used as indicator of environmental pollution and negative effects on physiological traits have to be considered.
Similar element concentrations in eggshells of addled eggs and eggshell remains from hatched eggs indicate no adverse effects of elevated Cu and Pb levels on hatching success of Common Cranes in Northern Germany. Similar levels of Cu and Pb in eggshells were found in Pied Flycatchers (Ficedula hypoleuca) across Europe, also showing no negative effect on hatching success (Ruuskanen et al. 2014). Elemental concentrations might differ between eggshell material and egg content or embryo, respectively, with varying ratios across different heavy metals. Whereas concentrations of Cu were found to be significantly higher in the egg content, compared to eggshells, Pb was found to be more equally distributed in eggshells and contents in several species (e.g., Orłowski et al. 2017; Thongcharoen et al. 2018; Ashkoo et al. 2020). Other authors found concentrations of inorganic elements, including Pb, to be significantly higher in eggshells compared to egg contents (e.g., Mora 2003). Considering this, further studies on elemental distributions in eggs of cranes are necessary to enable a reliable comparison of concentrations in eggshell and content. Moderate concentrations of Cu and Pb, like those found in our study, are not considered to be related to eggshell thinning in cranes (Luo et al. 2016). However, increased loads of non-essential elements, especially Cd and Pb, have been linked to deficiencies of essential elements, e.g., calcium (Ca), Cu, or Zinc (Zn), negatively effecting physiological traits (Dauwe et al. 2006), but amounts of Pb discussed in this context are several times higher than concentrations found in our study.
Results indicate that heavy metal content of eggshells does not increase with increasing female age, but different than expected even seems to decrease with increasing female age for Pb. Studies on elemental concentrations in eggshells in relation to age are missing in long-living species. Gochfeld et al. (1996) found heavy metals accumulating within soft tissues, which has been confirmed in birds of the family Gruidae by Teraoka et al. (2007), but precise mechanism behind the deposition of heavy metals in eggshells remain unknown. Once again, the question arises whether cranes are able to effectively excrete heavy metals through feather growth or incorporation in bones, respectively, or if physiological capabilities of excreting harmful substances decrease during ageing processes. The wide range of metal concentrations detected in eggshells in various species contradict the hypothesis of a physiological limitation of the total amount of heavy metals deposited in eggshells (e.g., Orłowski et al. 2017; Thongcharoen et al. 2018), but effects, including age dependency, might be species-specific. Studies on accumulation of pollutants in wild birds over longer periods of time are scarce, especially concerning concentrations of heavy metals in soft tissues. Other than sampling blood, investigations of soft tissues, such as kidney or liver, are highly invasive and multiple sampling occasions for single individuals are difficult. Studies on Great Skuas (Catharacta skua) found no relation between age and Hg levels in feathers or soft tissue, despite increasing mercury pollution (Thompson et al. 1991).
We also lack information on the proportions of toxic substances deposited in feathers and eggs within one individual. Only few studies investigating heavy metal levels in eggs and feathers from the same individuals exist. Burger and Gochfeld (1996) found heavy metal levels to be significantly lower in eggshells of Franklin's Gull (Larus pipixcan) compared to feathers of the parents. Similarly, levels of Cu in feathers of Bridled Terns (Onychoprion anaethetus) and Black-naped Terns (Sterna sumatrana) were found to be significantly higher compared to those of eggshells, but sampling took place on a random basis. In addition, individual variation of Cu contamination was very high ranging from 4.13 to 25.80 µg/g. Contrary, levels of Cd and Pb were shown to be more or less equal in eggs and feathers of these species (Hamza et al. 2021).
The decrease of Pb and Cu levels found in eggshells of Common Cranes over the years can be explained by multiple factors. The final ban of lead additives for gasoline in the early twenty-first century, and the reduction of heavy metal-based biocontrol products used in agriculture (Tamm et al. 2022) likely contributed to the reduction of Pb emissions significantly. Cu is an essential trace element and information on the amount of Cu naturally occurring in eggshells of cranes are lacking. Therefore, estimating the proportion of Cu originating from environmental pollution is difficult. Even though copper-based fungicides and pesticides have been shown to be ecologically harmful, they are still being used across Europe (Tamm et al. 2022). However, results do not clearly confirm our initial hypothesis that eggshells of cranes nesting within habitats surrounded by intensively used agricultural field show higher concentrations of heavy metals. We found no distinct link between heavy metal concentrations and landscape compositions of home ranges, but on a larger scale, landscape composition seems to have an effect. Levels of copper decrease in accordance with a decreasing proportion of agricultural fields from South to North within the study area. Elevated levels of Cu in eggshells from the southern part of the study area substantiate a link between intensive agriculture and elevated amounts of Cu in the environment. In addition, heavy metals from industrial processes, traffic or pesticides are persistent and accumulate in the environment over decades (Sotherton and Holland 2003), and material abrasion from automobiles and machinery still release heavy metals, especially Cu and Pb, into the environment. The related occurrence of these two elements might be explanatory for the correlation of Cu and Pb found in cranes’ eggshells. It has been shown that heavy metal concentrations in eggs (shell and content), including Cd, Cu, Fe, Mn, Ni, and Pb, are positively correlated with metal levels found in surrounding environmental matrices verifying the suitability of eggshell material as bioindicator for heavy metal pollution (Ruuskanen et al. 2014; Tanhan et al. 2020).
Cranes, such as other phytophagous and omnivorous birds, were found to mainly ingest heavy metals through food items, such as plants and invertebrates, or water (Brown et al. 2018; Xia et al. 2021). Concentrations of Cu detected in eggshells of Common Cranes are similar to those found in eggshells of Red-crowned Cranes from Northeastern China (ranging from ND to 8.05 ppm; Luo et al. 2016), whereas levels of Pb we found were markedly lower compared to those found in eggshells of Red-crowned Cranes (ranging from ND to 2.09 ppm; Luo et al. 2016). A higher proportion of industrial facilities within the home range of Red-crowned Cranes is considered explanatory. The ingestion of lead particles from leaded ammunition or fishing accessories has been reported in Red-crowned Cranes (Teraoka et al. 2007), Sandhill Cranes (Grus canadensis; Windingstad et al. 1984), and Whooping Cranes (Snyder et al. 1992), resulting in lethal Pb intoxications. Given that reports on lead poisoning in Common Cranes are missing, ammunition or fishing weights are not considered a major source of Pb detected in eggshells included in this study. However, lead being emitted into the environment from ammunition or fishing accessories and accumulating in the food chain can not be ruled out within the study area.
Differences in heavy metal content in accordance with the dominant vegetation can most likely be explained by differences between plant species’ capacities to absorb and accumulate them. Increasing levels of heavy metals have been found to be positively correlated with overall eutrophication of wetlands (Dreshaj et al. 2016). Species of the genus Typha are known to be highly competitive wetland species coping extraordinarily well with high nutrient levels (e.g., Motivans and Apfelbaum 1987; Boers et al. 2007; Pandey and Verma 2018), which could be an indicator of high eutrophication statuses of wetlands that are dominated by these species. However, species of the genus Typha, especially T. latifolia, which represents the most common Typha species present in our study area, are also known to have a high absorption capacity and accumulation rate of heavy metals, including Pb (Chiţimus et al. 2016). Researchers even suggest using Typha spec. for heavy metal removal from sewage and industrial wastewaters (Syukor et al. 2016; Bokhari et al. 2017). High metal absorption rates of Typha spec. lower overall Pb levels at nesting sites of cranes, e. g. in water or insect food items, reducing the probability of Pb intake by cranes. Contrary, S. cinerea was found to show lower heavy metal accumulation, especially under wet conditions (Vandecasteele et al. 2005), assuming higher heavy metal availability in the environment at habitats dominated by S. cinerea.
Availability and bioaccessibility of heavy metals in the environment are also affected by geo-chemical processes, explaining weather dependency of metal levels detected (Nriagu 1974; Grecco et al. 2010). The reduction of Pb in eggshells with increasing precipitation might result from acid rain, occurring globally (Grennfelt et al. 2020). Acidification promotes the mobilization of phosphorus (P), which then interacts with accessible Pb in the soil (Jalali and Naderi 2012). Due to its low solubility, lead–phosphates are mainly not available to plants and, therefore, do not enter the food chain (Nriagu 1974). Complementing, severe drought events have been linked to increased availability of Pb in sediments (Costa et al. 2020). Low temperatures slow down chemical processes and reduce trace element mobility (Sherene 2010), probably being accountable for a reduced bioavailability of Cu following strong winters. To be accessible for plants and other organisms, Cu needs to be available as water-soluble fraction or complexed with e.g., organic matter (Romić et al. 2013).
Arsenic and cadmium
Neither As nor Cd was found in any of the samples analysed. The absence of As in eggshells of these Common Cranes could be explained by the landscape and economic features of the study area. Besides the natural occurrence of As in certain rock types, the main sources of As in our environment have been arsenical pesticides and emissions during industrial processes (German Environment Agency 2023). High levels of As found in eggshells of Rooks (Corvus frugilegus) from Poland were found to be linked to a high usage of pesticides (Orłowski et al. 2010). However, due to the high toxicity of As, the use of pesticides containing As in Germany has been limited in 1942 and completely banned in 1974 (Brandt et al. 2010), explaining the low availability of As in the environment. In addition, arsenical pesticides have mainly been used for viticulture (Brandt et al. 2010), of which our study area is not considered a key growing area (Federal Statistical Office 2022). The federal state of Mecklenburg–Western Pomerania also represents the state with the lowest industrial activity within Germany (Braun et al. 2013). Considering this, results confirm our initial expectations to not find considerable amounts of As and Cd in eggshells of Common Cranes. The usage of Cd compounds in pesticides has been banned in 1974, as well. Studies on White-tailed Eagles (Haliaeetus albicilla) from Germany also found no or only very low concentrations of Cd in soft tissues (Kenntner et al. 2000), confirming our findings. However, several studies recently found detectable levels of Cd in eggshells as well as egg content in wild birds (Fu et al. 2013; Ashkoo et al. 2020), including cranes (Luo et al. 2016). Accordingly, we assume no substantial Cd pollution within our study area.
Conclusion
The population of Common Cranes studied is exposed to elevated levels of Cu and Pb, most likely resulting from agricultural practices, traffic, and industrial emissions. Concentrations found do not suggest negative effects on hatching success or embryonic development. However, since metal concentrations of blood and soft tissues of egg-laying females are believed to be several times higher than those of eggshells, it can be assumed that single adult individuals might suffer heavy metal contamination, considered to be potentially harmful. Future studies on heavy metal presence in cranes in Germany should include other tissues, e.g., blood, soft tissues, and feathers, as well as environmental samples, e.g., water, soil, plants, to extend the knowledge on both, the species heavy metal burden and general aspects of heavy metal contamination in the context of a species biology and ecology. Our investigation may be considered as preliminary study on the heavy metal contamination status of Common Cranes in Germany, giving first insights into the potential usage of eggshell residues of Common Cranes as bioindicator for monitoring environmental pollution.
Data availability
The data sets generated during and/or analysed during the current study are not publicly available for species conservation reasons but are available from the corresponding author on reasonable request.
References
Almalki AM, Ajarem J, Allam AA, El-Serehy HA, Maodaa SN, Mahmoud AM (2019) Use of Spilopelia senegalensis as a biomonitor of heavy metal contamination from mining activities in Riyadh (Saudi Arabia). Animals 9:1046
Ashkoo A, Amininasab SM, Zamani-Ahmadmahmoode R (2020) Bioaccumulation of heavy metals in eggshell and egg content of seabirds: lesser (Thalasseus bengalensis) and Greater Crested Tern (Thalasseus bergii). Mar Pollut Bull 154:111126
Bates DM, Maechler M, Bolker BM, Walker S (2015) Fitting linear mixed-effects models using lme4. J Stat Softw 67:1–48
Boers AM, Veltman RLD, Zedler JB (2007) Typha x glauca dominance and extended hydroperiod constrain restoration of wetland diversity. Ecol Eng 29(3):232–244
Bokhari SH, Mahmood-ul-Hassan M, Riaz Y, Munir A, Ali Z (2017) Baseline water quality of municipal ponds and metal removal ability of Typha latifolia L. from sewage and industrial wastewaters. Int J Phytoremediat 19:1077–1084
Brandt P, Bonath K, Joermann G (2010) Berichte zu Pflanzenschutzmitteln 2009—Wirkstoffe in Pflanzenschutzmitteln Zulassungshistorie und Regelungen der Pflanzenschutz-Anwendungsverordnung. Federal Office of Consumer Protection and Food Safety (BVL). Springer Science + Business Media, Basel, Switzerland. https://www.bvl.bund.de/SharedDocs/Downloads/04_Pflanzenschutzmittel/bericht_WirkstoffeInPSM_2009.pdf?__blob=publicationFile&v=3. Accessed 6 June 2023
Braun G, Güra T, Henn S, Lang T, Schürmann C, Voß K, Warszycki P (2013) Atlas der Industrialisierung der Neuen Bundesländer. Hanseatic Institute for Entrepreneurship and Regional Development, University of Rostock. https://www.igmetall-perspektive-ost.de/fileadmin/user/Dokumente/2013-11_Atlas_der_Industrialisierung_der_neuen_Bundeslaender.pdf. Accessed 6 June 2023
Brown L, Rosabal M, Sorais M, Poirier A, Widory D, Verreault J (2018) Habitat use strategy influences the tissue signature of trace elements including rare earth elements in an urban-adapted omnivorous bird. Environ Res 168(261):269
Burger J (1994) Heavy metals in avian eggshells: another excretion method. J Toxicol Environ Health 41:207–220
Burger J, Gochfeld M (1996) Heavy metal and selenium levels in Franklin’s gull (Larus pipixcan) parents and their eggs. Arch Environ Contam Toxicol 30:487–491
Chen M, Qin X, Zeng G, Li J (2016) Impacts of human activity modes and climate on heavy metal “spread” in groundwater are biased. Chemosphere 152:439–445
Chiţimus AD, Radu C, Nedess V, Moşnegutu E, Bârsan N (2016) Studies and researches on Typha Latifolia’s (Bulrush) absorption capacity of heavy metals from the soil. St Cerc St CICBIA 17:381–393
Costa ES, Sa F, Gomes LEO, Silva CA, Lima AT, Lehrback BD, Neto RR (2020) Can severe drought periods increase metal concentrations in mangrove sediments? A case study in eastern Brazil. Sci Total Environ 748:142443
Dauwe T, Snoeijs T, Bervoets L, Blust R, Eens M (2006) Calcium availability influences lead accumulation in a passerine bird. Anim Biol 56:289–298
Dreshaj A, Millaku B, Selimaj A, Feka F, Kelmendi M (2016) Heavy metals in waters, penetrating the food, ecosystems and the economy of Kosovo. In: CBU international conference on innovations in science and education, Prague, pp 48–55
DWD (2018) Climate Data Center. German Meteorological Service. https://www.dwd.de/DE/leistungen/cdc_portal/cdc_portal.html
Fox J, Weisberg S (2019) An R companion to applied regression, 3rd edn. Sage, Thousand Oaks. https://socialsciences.mcmaster.ca/jfox/Books/Companion/. Accessed 18 Aug 2022
Franson JC, Pain DJ (2011) Lead in birds. In: Beyer WN, Meador JP (eds) Environmental contaminants in biota—interpreting tissue concentrations, 2nd edn. CRC Press, Taylor, Francis Group, Boca Raton, pp 563–593
Federal Statistical Office (2022) Vines cultivated with wine grapes in 2022. Federal Statistical Office, Wiesbaden. https://www.destatis.de/EN/Themes/Economic-Sectors-Enterprises/Agriculture-Forestry-Fisheries/Wine/Tables/vine-acreage-laender.html. Accessed 6 June 2023
Fu J, Wang Q, Wang H, Yu H (2013) Monitoring of non-destructive sampling strategies to assess the exposure of avian species in Jiangsu Province, China to heavy metals. Environ Sci Pollut R 21:2898–2906
Fuchs S, Scherer U, Wander R, Behrendt H, Venohr M, Opitz D, Hillenbrand T, Marscheider-Weidemann F, Götz T (2010) Calculation of emissions into rivers in Germany using the MONERIS Model—Nutrients, heavy metals and polycyclic aromatic hydrocarbons Bundesumweltamt, Federal Environment Agency (Germany). https://www.umweltbundesamt.de/sites/default/files/medien/461/publikationen/4018.pdf. Accessed 24 Apr 2022
Furness RW (1993) Birds as monitors of pollutants. In: Furness RW, Greenwood JJD (eds) Birds as monitors of environmental change. Springer, Dordrecht. https://doi.org/10.1007/978-94-015-1322-7_3
German Environment Agency (2023) Schwermetall-Emissionen. Available at: https://www.umweltbundesamt.de/daten/luft/luftschadstoff-emissionen-in-deutschland/schwermetall-emissionen#entwicklung-seit-1990. Accessed 6 June 2023
Gochfeld M, Belant JL, Shukla T, Benson T, Burger J (1996) Heavy metals in Laughing gulls: gender, age and tissue differences. Environ Toxicol Chem 15:2275–2283
Gonzales LM, Hiraldo F (1988) Organochlorine and heavy metal contamination in the eggs of the Spanish Imperial Eagle (Aquila (heliaca) adalberti) and accompanying changes in eggshell morphology and chemistry. Environ Pollut 51:241–258
Grecco LE, Gómez EA, Botté SE, Marcos ÁO, Marcovecchio JE, Cuadrado DG (2010) Natural and anthropogenic heavy metals in estuarine cohesive sediments: geochemistry and bioavailability. Ocean Dynam 61:285–293
Grennfelt P, Engleryd A, Forsius M, Hov Ø, Rodhe H, Cowling E (2020) Acid rain and air pollution: 50 years of progress in environmental science and policy. Ambio 49:849–864
Hamza A, Hisham AS, Suratman S, Bidai JA, Shazili NAM (2021) Trace elements in feathers and eggshells of two tropical seabirds from Malaysia. Mar Ornithol 49:335–341
Höltje H, Mewes W, Haase M, Schmitz Ornés A (2016) Genetic evidence of female specific eggshell colouration in the Common Crane (Grus grus). J Ornithol 157:609–617
ICH—International Council on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use (2022) ICH Guideline Q2(R2) on validation of analytical procedures. European Medicines Agency
Jalali M, Naderi E (2012) The impact of acid rain on phosphorus leaching from a sandy loam calcareous soil of western Iran. Environ Earth Sci 66:311–317
Jeng SL, Lee J, Liu YF, Yang SC, Liou PP (1997) Effect of lead ingestion on concentrations of lead in tissues and eggs of laying Tsaiya Ducks in Taiwan. Poultry Sci 76:13–16
Jiao W, Ouyang W, Hao FH, Wang FL, Liu B (2014) Long-term cultivation impact on the heavy metal behaviour in a reclaimed wetland, Northeast China. J Soils Sedim 14:567–576
Kenntner N, Tataruch F, Krone O (2000) Heavy metals in soft tissues of White-tailed eagles found dead or moribund in Germany and Austria from 1993 to 2000. Environ Toxicol Chem 20:1831–1837
Kertész V, Bakonyi G, Farkas B (2006) Water pollution by Cu and Pb can adversely affect mallard embryonic development. Ecotoxicol Environ Safe 65:67–73
Kraus K, Römer J (2019) Schwermetalle—40 Jahre Genfer Luftreinhaltekonvention – Minderung von Cadmium, Blei und Quecksilber. Federal Environment Agency (Germany). https://www.umweltbundesamt.de/sites/default/files/medien/376/publikationen/uba_flyer_dt_schwermetalle_191128.pdf. Accessed 6 June 2023
Kumar PS, Yaashikaa PR (2019) Agriculture pollution. In: Advanced treatment techniques for industrial wastewaster. IGI Global, Hershey, pp 134–154
Lewis CJ, Drewien RD, Kuyt E, Sanchez C Jr. (1992) Contaminants in habitat, tissues, and eggs of whooping cranes. In: North American crane workshop proceedings, p 266
Lin YP, Anthony J, Mukhtar H, Lin CM (2021) A spatial prioritization method for identifying potential eco-risk distributions of heavy metals in soil and birds. Ecotoxicol Environ Safe 220:112383
Lüdecke D, Ben-Shachar MS, Patil I, Waggoner P, Makowski D (2021) Performance: an R package for assessment, comparison and testing of statistical models. J Open Source Softw 6:3139
LUNG (2010) Biotop- und Nutzungstypenkartierung (BNTK). Provided by the Landesamt für Umwelt, Naturschutz und Geologie (LUNG)
Luo J, Yin X, Yajie Y, Wang Y, Zang S, Zhou X (2013) Pb and Cd bioaccumulations in the habitat and prey of red-crowned cranes (Grus japonensis) in Zhalong Wetland, Northeastern China. Biol Trace Elem Res 156:134–143
Luo JM, Ye YJ, Gao ZY, Wang WF (2016) Trace element enrichment in the eggshells of Grus japonensis and its association with eggshell thinning in Zhalong Wetland (Northeastern China). Biologia 71:220–227
Martínez A, Crespo D, Fernández JA, Aboal JR, Carballeira A (2012) Selection of flight feathers from Buteo buteo and Accipiter gentilis for use in biomonitoring heavy metal contamination. Sci Total Environ 425:254–261
Martín-Fernández JA, Barceló-Vidal C, Pawlowsky-Glahn V (2003) Dealing With Zeros and Missing Values in Compositional Data Sets Using Nonparametric Imputation. Math Geol 35(3):253–278
Mewes W (2010) Population development, range distribution and population density of Common Cranes Grus grus in Germany and its federal states. Vogelwelt 131:75–92
Mewes W (2014) Die Bestandsentwicklung, Verbreitung und Siedlungsdichte des Kranichs Grus grus in Mecklenburg-Vorpommern von 1967 bis 2013. Ornithol Rundbrief Mecklenburg-Vorpommern 48:29–34
Mewes W (2017) Die Brutorttreue von Kranichen Grus grus in Nordostdeutschland. Vogelwelt 137:249–260
Mewes W, Rauch M (2010) Identification of breeding female Common Cranes Grus grus through their clutches. Vogelwelt 131:93–102
Monclús L, Shore RF, Krone O (2020) Lead contamination in raptors in Europe: a systematic review and meta-analysis. Sci Total Environ 748:141437
Mora MA (2003) Heavy metals and metalloids in egg contents and eggshells of passerine birds from Arizona. Environ Pollut 125:393–400
Morin-Crini N, Lichtfouse E, Liu GR, Balaram V, Ribeiro ARL, Lu ZJ, Stock F, Carmona E, Teixeira MR, Picos-Corrales LA, Moreno-Pirajan JC, Giraldo L, Li C, Pandey A, Hocquet D, Torri G, Crini G (2022) Worldwide cases of water pollution by emerging contaminants: a review. Environ Chem Lett. https://doi.org/10.1007/s10311-022-01447-4
Motivans K, Apfelbaum S (1987) Element stewardship abstract for Typha spp., North American Catttails. The Nature Conservancy, Arlington
Nriagu JO (1974) Lead orthophosphates—IV. Formation and stability in the environment. Geochem Cosmochim Acta 38:887–898
Orłowski G, Kasprzykowski Z, Dobicki W, Pokorny P, Polechoński R (2010) Geographical and habitat differences in concentrations of copper, zinc and arsenic in eggshells of the Rook Corvus frugilegus in Poland. J Ornithol 151:279–286
Orłowski G, Hałupka L, Pokorny P, Klimczuk E, Sztwiertnia H, Dobicki W, Polechoński R (2017) The pattern of distribution and interaction of metals and calcium in shells and egg contents in relation to the embryonic development of eggs in a small passerine bird. J Ornithol 158:297–309
Pandey A, Verma RK (2018) Taxonomical and pharmacological status of Typha: a review. Ann Plant Sci 7(3):2101–2106
Penkała M, Ogrodnik P, Rogula-Kozłowska W (2018) Particulate matter from the road surface abrasion as a problem of non-exhaust emission control. Environments. https://doi.org/10.3390/environments5010009
Prange H, Mewes W, Winter S (2016) Fortpflanzung und Jungenaufzucht. In: Prange H (ed) Die Welt der Kraniche. Leben—Umfeld—Schutz. Verbreitung aller Arten. MediaNatur Verlag Hans-Josef Christ, Minden
Rajput RS, Pandey S, Bhadauria S (2017) Status of water pollution in relation to industrialization in Rajasthan. Rev Environ Health 32:245–252
Rattner BA (2009) History of wildlife toxicology. Ecotoxicology 18:773–783
Romić M, Matijević L, Bakić H, Romić D (2013) Copper accumulation in vineyard soils: distribution, fractionation and bioavailability assessment. In: Hernandez-Soriano MC (ed) Environmental risk assessment of soil contamination. IntechOpen, London
Ruuskanen S, Laaksonen T, Morales J, Moreno J, Mateo R, Belskii E, Bushuev A, Järvinen A, Kerimov A, Krams I, Morosinotto C, Mänd R, Orell M, Qvarnström A, Slater F, Tilgar V, Visser ME, Winkel W, Zang H, Eeva T (2014) Large-scale geographical variation in eggshell metal and calcium content in a passerine bird (Ficedula hypoleuca). Eviron Sci Pollut Res 21:3304–3317
Sager M (2006) Selenium in agriculture, food, and nutrition. Pure Appl Chem 78(1):111–133
Schmitz Ornés A, Herbst A, Spillner A, Mewes W, Rauch M (2014) A standardized method for quantifying eggshell spot patterns. J Field Ornithol 85:397–407
Sherene T (2010) Mobility and transport of heavy metals in polluted soil environment. Biol Forum 2: 112–121
Snyder SB, Richard MJ, Thilsted JP, Drewien RC (1992) Lead poisoning in a whooping crane. Technical report 12. In Proceedings, 1988 North American Crane Workshop, Nongame Wildlife Program, February 22–24; Tallahassee: Florida Fish and Wildlife Conservation Commission, pp 207–210
Sotherton N, Holland J (2003) Indirect effects of pesticides on farmland wildlife. In: Hoffman DJ, Barnett A, Rattner G, Burton GA, Cairns J (eds) Handbook of ecotoxicology, 2nd edn. CRC Press, Boca Raton, pp 1173–1192
Spahn SA, Sherry TW (1999) Cadmium and lead exposure associated with reduced growth rates, poorer fledging success of Little Blue Heron Chicks (Egretta caerulea) in South Louisiana Wetlands. Arch Environ Contam Toxicol 37:377–384
Spallholz JE, Hoffman DJ (2002) Selenium toxicity: cause and effects in aquatic birds. Aquat Toxicol 57:27–37
Syukor ARA, Sulaiman S, Siddique MNI, Zularisam AW, Said MIM (2016) Integration of phytogreen for heavy metal removal from wastewater. J Clean Prod 112:3124–3131
Tamm L, Thuerig B, Apostolov S, Blogg H, Borgo E, Corneo PE, Fittje S, de Palma M, Donko A, Experton C, Alcázar Marín É, Morell Pérez Á, Pertot I, Rasmussen A, Steinshamm H, Vetemaa A, Willer H, Herforth-Rahmé J (2022) Use of copper-based fungicides in organic agriculture in twelve European countries. Agronomy 12:673
Tanhan P, Apipongrattanasuk N, Poapolathep A, Paopolathep S, Kruatrachue M, Imsilp K (2020) Heavy metal concentrations in duck eggs and potential human health risk via consumption. Jpn J Vet Res 68:21–33
Teraoka H, Kumagai Y, Iwai H, Haraguchi K, Ohba T, Nakai K, Satoh H, Sakamoto M, Momose K, Masatomi H, Hiraga T (2007) Heavy metal contamination status of Japanese cranes (Grus japonensis) in east Hokkaido, Japan—extensive mercury pollution. Environ Toxicol Chem 26:307–312
Thompson DR, Hamer KC, Furness RW (1991) Mercury accumulation in great skuas Catharacta Skua of known age and sex, and its effects upon breeding and survival. J Appl Ecol 28:672–684
Thongcharoen K, Robson MG, Keithmaleesatti S (2018) Determination of heavy metals in eggs of Little Grebe (Tachybaptus ruficollis) around the wastewater treatment ponds, Khon Kaen University. Hum Ecol Risk Assess 24:362–376
Tumanyan AF, Seliversova AP, Zaitseva NA (2020) Effects of heavy metals on ecosystems. Chem Tech Fuels Oil 56:390–394
Ullah AKMA, Maksud MA, Khan SR, Lutfa LN, Quraishi SB (2017) Development and validation of a GF-AAS method and its application for the trace level determination of Pb, Cd, and Cr in fish feed samples commonly used in the hatcheries of Bangladesh. J Anal Sci Technol 8:1–7
Urbina I, Sardans J, Beierkuhnlein C, Jentsch A, Backhaus S, Grant K, Kreyling J, Peñuelas J (2015) Shifts in the elemental composition of plants during a very severe drought. Environ Exp Bot 111:63–73
Vandecasteele B, Quataert P, Tack FMG (2005) The effect of hydrological regime on the metal bioavailability for the wetland plant species Salix cinerea. Environ Pollut 135:303–312
Wayland M, Scheuhammer AM (2011) Cadmium in birds. In: Beyer WN, Meador JP (eds) Environmental contaminants in biota—interpreting tissue concentrations, 2nd edn. CRC Press Taylor, Francis Group, Boca Raton, pp 645–666
Windingstad RM, Kerr SM, Locke LN (1984) Lead poisoning of sandhill cranes (Grus canadensis). Prairie Nat 16:21–24
Xia P, Ma L, Yi Y, Lin T (2021) Assessment of heavy metal pollution and exposure risk for migratory birds—a case study of Caohai wetland in Guizhou Plateau (China). Environ Pollut 275:116564
Zhang WW, Ma JZ (2011) Waterbirds as bioindicators of wetland heavy metal pollution. Proc Environ Sci 10:2769–2774
Acknowledgements
We are grateful to all people who supported the sample collection in the field over many years, especially Moriz Rauch and Volker Mewes. We thank Dr. Silke Fregin for her vigorous support and assistance in the lab during sample preparation. In addition, we thank Dr. Daniel Bäcker for assistance with the atomic absorption spectrometer.
Funding
Open Access funding enabled and organized by Projekt DEAL. Acquisition of consumable supplies needed for AAS was partly funded by the German Ornithologists' Society (DO-G, Wilhelmshaven, Germany).
Author information
Authors and Affiliations
Contributions
Conceptualization: IB (main/equal), WM (main/equal), ASO (support), SG (support); field work and sample collection: WM (main), IB (support); methodology: IB (equal), SG (equal); sample analyses: IB (main), SG (support); writing—original draft preparation: IB; writing—review and editing: ASO (main/equal), SG (main/equal), WM (support); funding acquisition: IB (equal), ASO (equal), SG (equal), supervision; SG (main), ASO (support). All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
All authors confirm that they have no affiliations with or involvement in any organization or entity with any financial interest, or non-financial interest in the matter discussed in this manuscript.
Ethics approval and consent to participate
No approval of research ethics committees was required to accomplish the goals of this study, because no animal was involved.
Additional information
Communicated by F. Bairlein.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Barwisch, I., Mewes, W., Schmitz Ornés, A. et al. Heavy metal residues in eggshells of Common Cranes (Grus grus) nesting in an agricultural region in north-eastern Germany. J Ornithol 165, 507–520 (2024). https://doi.org/10.1007/s10336-023-02122-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10336-023-02122-3