Abstract
Camera traps are increasingly used to estimate the density of animals as well as their activity patterns. As camera traps allow monitoring of animals over long periods of time without disturbance, they are especially useful to observe changes in diurnal activity patterns over time. In ornithology, camera trapping is still in its infancy. To our knowledge, no study has yet investigated the activity pattern of a songbird over the full annual cycle. We used camera traps in the Rammert, a small mountainous forest area near Rottenburg in Southwest Germany to monitor the diurnal activity pattern of forest-dwelling Eurasian blackbirds (Turdus merula). As the activity level of animals is known to be affected by day light, we used double-anchoring transformation of day times to account for the variation in sunrise and sunset across the different seasons. By generating activity models, we investigated the pattern of blackbird activity during the four seasons of the year and compared the patterns of male and female birds, respectively. A significant difference between a unimodal activity pattern in spring and a bimodal pattern for the rest of the year was found which might be related to breeding and territorial behaviour in spring. Moreover, we observed that the activity pattern of males and females overlapped greatly but still showed some variation in the number and timing of density peaks.
Zusammenfassung
Saisonale Variation der Tagesaktivität von Amseln (Turdus merula) im Wald
Kamerafallen werden zunehmend eingesetzt, um die Dichte von Tieren und ihre Aktivitätsmuster zu ermitteln. Da Kamerafallen die Beobachtung von Tieren über lange Zeiträume hinweg ermöglichen, ohne sie zu stören, sind sie besonders nützlich, um Veränderungen in den täglichen Aktivitätsmustern im Laufe der Zeit zu beobachten. In der Ornithologie steckt die Kamerafallennutzung allerdings noch in den Kinderschuhen. Unseres Wissens wurde bisher in keiner Studie das Aktivitätsmuster eines Singvogels über den gesamten Jahreszyklus hinweg untersucht. Wir haben Kamerafallen im Rammert, einem kleinen Mittelgebirgswald bei Rottenburg im Südwesten Deutschlands, eingesetzt, um das tageszeitliche Aktivitätsmuster von waldbewohnenden Amseln (Turdus merula) zu beobachten. Da das Aktivitätsniveau der Tiere durch Tageslicht/Photoperiode beeinflusst wird, verwendeten wir eine doppelt verankerte Transformation der Tageszeiten, um die Variation von Sonnenaufgang und Sonnenuntergang in den verschiedenen Jahreszeiten zu berücksichtigen. Durch die Erstellung von Aktivitätsmodellen untersuchten wir das Muster der Amselaktivität während der vier Jahreszeiten und verglichen die Muster von männlichen bzw. weiblichen Amseln. Es wurde ein signifikanter Unterschied zwischen einem unimodalen Aktivitätsmuster im Frühjahr und einem bimodalen Muster für den Rest des Jahres festgestellt, was mit dem Brut- und Territorialverhalten im Frühjahr zusammenhängen könnte. Außerdem stellten wir fest, dass sich die Aktivitätsmuster von Männchen und Weibchen stark überschnitten, aber dennoch eine gewisse Variation in der Anzahl und dem Zeitpunkt der Dichtespitzen aufwiesen.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Niche partitioning, the process of using the environment in a unique way to enable coexistence of various species, also occurs on the scale of time. The resulting species-specific activity patterns are mostly determined by available illumination and suitability of thermal conditions (Bennie et al. 2014), resulting in a crepuscular, nocturnal, diurnal or cathemeral activity with uni- or multimodal distribution. However, the activity of individuals might be further affected by inter- and intraspecific interactions (Blanchet et al. 2008; Pita et al. 2011; Randler and Kalb 2020), the diurnal activity of prey or predators (Harmsen et al. 2011; Foster et al. 2013); food availability (Boulos and Terman 1980; Orpwood et al. 2006; Pereira 2010) or unfavourable environmental conditions such as weather events or poor light conditions (Fernandez-Duque 2003; Halle and Stenseth 2012).
Camera traps enable researchers to monitor animal behaviour over long time periods with minimal disturbance. The data collected can be used to investigate a variety of research questions ranging from species composition to population size estimates to animal behaviour such as diel activity patterns (Rovero et al. 2013; Fancourt 2016; Krauss et al. 2018; Randler et al. 2020). The number of studies using camera traps increased tremendously over the last years (Zimmermann et al. 2016; Wearn and Glover-Kapfer 2019). However, most studies aimed to capture large mammals, fewer investigated small mammals, and even less studies drew upon reptiles or birds (Burton et al. 2015; Krauss et al. 2018; Richardson et al. 2018; Randler and Kalb 2020). This bias is most likely due to a lower capturing success of small animals by the sensors of most camera traps as they might quickly move out of view before a photo can be taken. Moreover, small species are often hard to identify on images, especially if they are far away from the camera trap or the image quality is low due to e.g. weather or light conditions. Fortunately, there is a growing body of research concerning the sensitivity of camera traps and guidelines to optimise the usage of camera traps for monitoring small mammals and birds (Di Cerbo and Biancardi 2013; Hobbs and Brehme 2017; Randler and Kalb 2018; Dundas et al. 2019). By following the published guidelines (Di Cerbo and Biancardi 2013; Hobbs and Brehme 2017; Randler and Kalb 2018; Dundas et al. 2019), camera traps can be a powerful tool to observe activity patterns over days, weeks, months and even years as camera traps can collect data on a species presence for prolonged and sustained time periods.
The aim of this study was to investigate the activity pattern of forest-dwelling Eurasian blackbirds (Turdus merula, LINNÉ 1758) under natural environmental conditions throughout a whole year using camera trapping in the Rammert (48° 26′ 38.0" N 8° 58′ 59.5" E), a mainly forested ridge in the Keuper uplands near Rottenburg am Neckar, Southwest Germany. We investigated both the activity patterns of blackbirds throughout the year and sex-specific differences. These sex-specific differences were expected for example due to contrasting behaviour in spring and summer where males spend a considerable amount of time singing on elevated points and females are first building and later, sitting on the nest (Chamberlain et al. 1999; Post and Götmark 2006).
Materials and methods
Camera setup
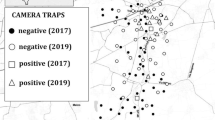
Basically, the guidelines of Meek et al. (2014) were followed utilising a somehow deliberately biassed placement. First, in order to spread the camera traps regularly within the study area, grids were used as a first orientation for choosing the camera trapping places. In a second step, cameras were placed within each grid at focal points to maximise detection (Meek et al. 2014). Trapping success is up to ten times higher when cameras were placed at trails or other structures rather than at random (Kolowski and Forrester 2017). A total of 21 camera traps were placed within 12 different 1 × 1 km large grids distributed in the northern part of the Rammert (Fig. 1). SecaCam Raptor cameras were used, which have been shown to be suitable for detecting small songbirds (Randler and Kalb 2018). To reduce autocorrelation effects (Dormann et al. 2007), cameras were placed in a distance of at least 300 m to each other. As we aimed to study forest-dwelling Eurasian blackbirds, cameras were applied in forested areas, and no camera was placed in the open landscape. Camera traps were placed between 362 and 552 m above sea level.
The recording started on the 13th of January 2021 and 17 cameras operated until 19th January 2022, and the rest had to be removed earlier due to technical difficulties. Camera #15 worked well but did not yield any images of blackbirds. Cameras were mounted on trees approximately about 60–80 cm above the ground. The surveyed areas covered different forest types and structural elements, such as dense underwood, small trails, dead wood, openings and grass-covered patches. The forest is formed mainly by Quercus spec., Picea abies, Fagus sylvatica and Pinus sylvestris. All camera traps operated for 24 h/d and collected a series of multiple photos when motion was detected. The starting date of each camera was defined as the date when the camera was deployed in the field. The end date was determined by the end of the survey or the last day the camera was proven to work. The date on the last triggered photo was used as end date. All camera traps were surveyed regularly to switch memory cards and batteries if necessary. The cameras are sensitive enough to take images during twilight and at night, and the cameras have been pretested to show that they are able to capture thrushes reliably (Randler and Kalb 2018). However, fast-flying birds cannot be recorded reliably by camera traps.
Activity analysis
As animals have to move to trigger a camera trap, we defined activity as movement of Eurasian blackbirds outside of refuges/nest or sleeping places and in front of our cameras (Rowcliffe et al. 2014). We further defined the clock time at which a camera was triggered as activity record. If birds repeatedly triggered the camera without leaving the field of view, only the initial trigger was used and defined as one blackbird event.
To analyse the activity patterns of blackbirds, we grouped the observations by seasons, namely spring from 1 March to 31 May, summer from 1 June to 31 August, and fall from 1 September to 30 November and winter from 1 December to 28 February. To account for the variation in day length throughout these three-month periods, a time transformation method suitable for 48.46° latitude was implemented. We transformed the times of the observations within each three-month period with a double-anchoring procedure as described by Vazquez et al. (2019). To apply this transformation to our data, we used the solar time function from the Animal Activity r-package (Rowcliffe et al. 2014; Rowcliffe 2023). The function transforms clock time to solar time anchored to the average sunrise and sunset times for a specific period and location (Rowcliffe 2023). During this transformation, the observation times get transformed into radian time format which is a numeric representation of clock times within the range of [0, 2*π].
After the transformation, we used the fitact function to fit kernel density functions and calculate activity levels for the transformed observation times (Vazquez et al. 2019). Whilst generating these activity models, we used bootstrapping to estimate errors. To obtain sensible confidence intervals despite dealing with low sample sizes for some seasons, we decided to sample from the fitted probability density distribution for the bootstrapping method instead of sampling from our data. We used these models to describe the changes of the activity patterns during the year. To understand more about the differences in activity patterns between male and female blackbirds, we grouped our data by sex and rerun the same computations.
To test for significant differences between all seasons, we first computed an NNTS likelihood ratio test for homogeneity in circular data as proposed by Fernández-Durán and Gregorio-Domínguez (2010). We then computed Watson's two-sided tests of homogeneity to determine the seasons for which differences exist and to detect differences between the sexes for specific seasons. In addition, we used the overlap package to determine the overlap of different kernel density estimations by calculating overlap coefficients and generating overlap plots for visualisation (Ridout and Linkie 2009; Meredith and Ridout 2021). We used the “Dhat1” overlap coefficient due to the small sample size (< 75), as recommended by Meredith and Ridout (2021).
All analyses were carried out with RStudio (RStudio Team 2020). Our data and a script to reproduce our results are available on reasonable request from the authors.
Results
Overall, 499 events of blackbirds (definition see above) were photographed by the camera traps. In spring, we recorded 147 blackbirds, 162 during summer, 123 in fall and 67 blackbirds during the winter period (Fig. 2). With one exception in April, we recorded more male than female blackbirds. Appendix Table 1 depicts the recorded observations for each camera trap.
The camera traps did not record nocturnal activity for blackbirds. As activity starts well before sunrise in all seasons but winter (when activity starts later), we assume a crepuscular–diurnal activity pattern for blackbirds. We found that the activity pattern of Eurasian blackbirds differs significantly throughout the year (Table 1).
Subsequent tests between different seasonal models showed significant differences between all seasons (Table 2).
Within all seasons, we identified 294 males (58.8%), 129 females (25.8%) and in 76 (15.2%) events, the sex of the blackbird could not be identified.
Although there were no significant differences between the raw activity data of both sexes (Appendix Table 2) throughout the seasons, the probability density functions of male and female blackbird activity look different (Fig. 3), and the overlap coefficient between activity is below 1 (Table 3).
Discussion
To our best knowledge, to date, no camera-trap-based study has been conducted to reveal the diurnal activity pattern of songbirds across the full annual cycle. Many factors are known to affect the diurnal activity pattern of animals, including illumination, food availability, brood care, and inter- and intraspecific interactions (Pita et al. (2011), Dominoni et al. (2014), Bogdan et al. (2016), Luo et al. (2019)). Therefore, we expected to find a difference in activity patterns between seasons as most of the previously mentioned factors, both biotic or intra- and interspecific relations and abiotic factors, fluctuate during the four seasons in Central Europe. Our study revealed significant differences of activity data between all seasons. Furthermore, in summer, fall and winter, we found a bimodal activity pattern that most probably reflects underlying basic diurnal rhythms (Santiago-Quesada et al. 2012).
Seasonal differences
For spring, we found a unimodal activity pattern which peaks after noon. Activity starts before sunrise and ends shortly after sunset (Fig. 4). The missing bimodality is conspicuous, and we assume that the longer photoperiod in spring in contrast to the darker winter is reflected in the activity pattern. Different reasons might explain this result. During spring, blackbirds might constantly allocate time to nest building, mating and breeding throughout the day and therefore may not show distinct activity peaks. Moreover, male blackbirds fly to their perches for singing during twilight early in the morning to establish a territory in early March (Hölzinger 1994). Dominoni et al. (2013a) showed that blackbirds from the forest started singing before twilight in the lab. However, the studies are difficult to compare because Dominoni et al. (2013b) studied the onset of song activity of blackbirds experimentally in the lab, whilst our study considered activity pattern under natural conditions, and thus did not explicitly collect data on song. The additional use of audio recording data could compensate for the shortcoming that with our camera trap setting it was not possible to record blackbirds early in the morning during spring, when males are singing up in the tree.
Blackbird diurnal activity pattern for spring (a), summer (b), fall (c) and winter (d). The step function depicts the frequency of black bird observations, i.e. the transformed raw data per time interval (red line). Our activity model consists of von Mises kernel distribution fitted to raw data (blue lines) with 95% confidence interval (dashed lines).The light grey bars indicate the average times for civil dawn respectively civil dusk for the observed season. The dark grey bars indicate average night hours. The lines indicate the average sunset and sunrise times. Times are in radian time format within the range of [0, 2*π]
Additionally, in spring, migrating blackbirds may mix up with the residents which may mask the pattern of diurnal activity since migrants may not sing and show a different behaviour than residents, because they have to refuel and restore body fat, or need more time for resting (Randler et al. 2015). Migrating Central European blackbirds have an earlier seasonal timing than northern populations. Thus, migrants from Scandinavia may also occur in our observations later in spring (Main 2002) which may influence the activity pattern in our data. As, there are only few published data about the phenology of the annual spring migration of blackbirds, further studies focussing on the springtime should be applied. Moreover, urban blackbirds have changed their migration behaviour to a residence status in many European regions as a response to climate change (Moller et al. 2014; van Vliet et al. 2009). Here, we studied forest-dwelling blackbirds that may differ from urban blackbirds. In our study, the lowest numbers of blackbirds were recorded between November and April. Thus, one part of the population of the Rammert might leave the forest to winter in urban areas, migrate to more southern regions. Alternatively, blackbirds may fly longer distances within the forest during the winter because territorial behaviour is absent during the winter months, whilst in spring, the birds are more confined to their territories. Bird detection probabilities should be even higher during the winter compared to summer because the camera traps are based on the temperature differences between moving objects and their surroundings, cooler winter days should elicit more photos compared to the warmer summer days (Randler and Kalb 2018). Further studies should address these differences between urban and forest blackbirds. Even differences concerning the activity pattern could be caused by the fact that urban populations start their daily activity earlier (Dominioni et al. 2013a) and show a longer activity period because of artificial lights at night (Russ et al. 2015). Apart from spring, blackbirds showed a clear activity pattern with a bimodal activity, as in many other animals, including humans (Santiago-Quesada et al. 2012).
The cameras detected fewer blackbirds during January and February. This may be caused by a lower density, a lower activity and less movement during that period. Various studies showed that temperature is an important variable concerning metabolism in passerine birds (Dawson and Marsh 1986; Lehikoinen 1987; Cresswell 1998; Swanson and Olmstead 1999; Gosler 2002; Rogers and Reed 2003). In addition to temperature, the shorter photoperiod during winter season also seems to be an important factor affecting foraging because of the time available for feeding (Haftorn 1989; Steinmeyer et al. 2010; Stuber et al. 2015; Schlicht and Kempenaers 2020). However, differences in activity patterns between seasons can be the result of behavioural responses to environmental conditions to which the birds are exposed (temperature, precipitation, etc.). Thus, those patterns may not be related to an underlying circadian rhythm. To test the underlying circadian rhythms, experiments are needed on a seasonal basis by exposing the animals to constant dim light conditions across the different seasons.
Sex differences
Besides changing abiotic factors, the activity pattern of blackbirds might further be affected by sex. However, a limitation of our study was that we could not identify the sex of blackbirds in 15.2% of all individuals, which is mostly owed to the occurrence of blackbirds around sunrise and sunset. In dark ambient conditions, the camera traps switch from colour to black and white photographs and use an infrared flash (blacklight), making it difficult to identify the sex of the individuals pictured. To solve this problem, camera traps with white flash could be used, but since this kind of flash is likely to severely disturb the animals, this practice could have a detrimental effect on the photo yield if the camera location is avoided as a result of the flashing at night (Henrich et al. 2020).
In passerines, the foraging patterns of males and females are known to differ as male foraging heights are often correlated with male song perch heights, whereas those of females are related to nest heights (Holmes 1986; Randler et al. 2010). Such differences in foraging behaviour could also result in different activity patterns captured by camera traps. Indeed, the study of (Post and Götmark 2006) revealed that female blackbirds foraged more and at lower heights than males.
The comparison of the activity pattern of males and females in our study revealed an overall large overlap of activity. However, males were active earlier than females in spring. During spring, males defend their territory vigorously against male intruders by singing on song posts and directly by territorial conflicts (own observations) which increases the chances of being trapped by a camera. Females, on contrast are breeding on the nest during this time of the day and therefore were recorded less. In summer, both sexes are more active before noon, that is, when the temperature is lower and humidity is higher, both beneficial to foraging earthworms. In fall, males show two distinct peaks in the morning and afternoon as well as one smaller peak right at noon, whereas females were most active around noon. Both sexes are more active in the afternoon in winter. A more extensive survey on individually marked blackbirds or a telemetry study might reveal the relationships between activity patterns and the effect of different factors, like predation risks, energy needs or parental investment throughout the year (Chamberlain et al. 1999; Post and Götmark 2006).
Limitations and methodological implications
The main disadvantage for camera trapping is that activity data is only collected for behaviour displayed in front of the camera traps, for example, foraging in leaf litter when the camera is placed accordingly. Other behaviour occurring out of view, such as singing on perches above the camera angle, will not be recorded and therefore not be included in the resulting activity profiles. This issue can partly be addressed by placing camera traps in all environments relevant to the respective species. Another problem was that sex determination and even determination between females and juveniles were not always possible due to image quality reasons. This is expected to be a smaller problem in the future as optical equipment has been continuously improved in recent years. A detailed result of an individual activity pattern could rather be achieved using invasive methods, such as radiotelemetry and automated bases stations using radio-frequency identification (RFID). For example, passive integrated transponders (PIT tags) can be read by RFID antennas, and these data can be automatically sampled, e.g. during feeder visits, and also, radio-tagging can be used (Santiago-Quesada et al. 2012; Hillemann et al. 2019; Randler 2021). Some recent studies used an array of digital radio-receiving stations (Gottwald et al. 2019; Karwinkel et al. 2022). On the one hand, the automated techniques have a high quality of activity data collection and allow individual identification, which is usually not the case for camera trapping. On the other hand, radio-tagging and radiotelemetry require the desired animal to be captured at least once, which might affect the normal behaviour and therefore, recorded activity. Furthermore, radio signals can be disturbed in dense forest areas and radio-receiving stations needs intensive maintenance.
A combination of both non-invasive methods, audio recordings and camera traps would likely reveal the most insights about the activity pattern of a certain species. The potential of camera traps to quantify approximate activity patterns of songbirds without the need to catch birds in the field is quite high because it does not need a permission/licence and it is also characterised by cheap devices, little workload in comparison to catching and fitting transmitters, and not time-consuming in acquisition.
Conclusion
Our study shows that camera trap surveys are useful to determine the activity pattern across all seasons of small songbirds such as the Eurasian blackbird. Camera traps can thereby be a useful tool to survey the activity pattern of a species over long time periods to identify possible changes in activity within day and season.
Data availability
The data used for this research is available from the corresponding author on reasonable request.
References
Bennie JJ, Duffy JP, Inger R, Gaston KJ (2014) Biogeography of time partitioning in mammals. Proc Natl Acad Sci 111:13727–13732
Blanchet S, Loot G, Bernatchez L, Dodson JJ (2008) The effects of abiotic factors and intraspecific versus interspecific competition on the diel activity patterns of Atlantic salmon (Salmo salar) fry. Can J Fish Aquat Sci 658:1545–1553
Bogdan V, Jůnek T, Vymyslická PJ (2016) Temporal overlaps of feral cats with prey and competitors in primary and human-altered habitats on Bohol Island, Philippines. PeerJ 4:e2288
Boulos Z, Terman M (1980) Food availability and daily biological rhythms. Neurosci Biobehav Rev 42:119–131
Burton AC, Neilson E, Moreira D, Ladle A, Steenweg R, Fisher JT, Bayne E, Boutin S (2015) Wildlife camera trapping: a review and recommendations for linking surveys to ecological processes. J Appl Ecol 523:675–685
Chamberlain D, Hatchwell B, Perrins C (1999) Importance of feeding ecology to the reproductive success of blackbirds Turdus merula nesting in rural habitats. Ibis 141:415–427
Cresswell W (1998) Diurnal and seasonal mass variation in blackbirds Turdus merula: consequences for mass-dependent predation risk. J Anim Ecol 671:78–90
Dawson WR, Marsh RL (1986) Winter fattening in the American Goldfinch and the possible role of temperature in its regulation. Physiol Zool 593:357–368
Di Cerbo AR, Biancardi CM (2013) Monitoring small and arboreal mammals by camera traps: effectiveness and applications. Acta Theriol 583:279–283
Dominoni DM, Helm B, Lehmann M, Dowse HB, Partecke J (2013a) Clocks for the city: circadian differences between forest and city songbirds. Proc Royal Soc B: Biol Sci 280:20130593
Dominoni D, Quetting M, Partecke J (2013b) Artificial light at night advances avian reproductive physiology. Proc Royal Soc B: Biol Sci 280:20123017
Dominoni DM, Carmona-Wagner EO, Hofmann M, Kranstauber B, Partecke J (2014) Individual-based measurements of light intensity provide new insights into the effects of artificial light at night on daily rhythms of urban-dwelling songbirds. J Anim Ecol 833:681–692
Dormann FC, McPherson M, Araújo JB, Bivand M, Bolliger R, Carl JG, Wilson R (2007) Methods to account for spatial autocorrelation in the analysis of species distributional data: a review. Ecography 30:609–628
Dundas SJ, Ruthrof KX, Hardy GESJ, Fleming PA (2019) Pits or pictures: a comparative study of camera traps and pitfall trapping to survey small mammals and reptiles. Wildl 462:104–113
Fancourt BA (2016) Avoiding the subject: the implications of avoidance behaviour for detecting predators. Behav Ecol Sociobiol 709:1535–1546
Fernandez-Duque E (2003) Influences of moonlight, ambient temperature, and food availability on the diurnal and nocturnal activity of owl monkeys (Aotus azarai). Behav Ecol Sociobiol 545:431–440
Fernández-Durán JJ, Gregorio-Domínguez MM (2010) A Likelihood ratio test for homogeneity in circular data. J Biomet Biostat 1:107. https://doi.org/10.4172/2155-6180.1000107
Foster VC, Sarmento P, Sollmann R, Tôrres N, Jácomo AT, Negrões N, Fonseca C, Silveira L (2013) Jaguar and puma activity patterns and predator-prey interactions in four Brazilian biomes. Biotropica 453:373–379
Gosler AG (2002) Strategy and constraint in the winter fattening response to temperature in the great tit Parus major. J Anim Ecol 715:771–779
Gottwald J, Zeidler R, Friess N, Ludwig M, Reudenbach C, Nauss T (2019) Introduction of an automatic and open-source radio-tracking system for small animals. Meth Ecol Evol 10:2163–2172
Haftorn S (1989) Seasonal and diurnal body weight variations in titmice, based on analyses of individual birds. Wilson Bull 101:217–235
Halle S, Stenseth NC (2012) Activity patterns in small mammals: an ecological approach. Springer
Harmsen BJ, Foster RJ, Silver SC, Ostro LE, Doncaster C (2011) Jaguar and puma activity patterns in relation to their main prey. Mamm Biol 763:320–324
Henrich M, Niederlechner S, Kröschel M, Thoma S, Dormann CF, Hartig F, Heurich M (2020) The influence of camera trap flash type on the behavioural reactions and trapping rates of red deer and roe deer. Remote Sens Ecol Conserv 63:399–410
Hillemann F, Cole EF, Keen SC, Sheldon BC, Farine DR (2019) Diurnal variation in the production of vocal information about food supports a model of social adjustment in wild songbirds. Proc Royal Soc B 286:20182740
Hobbs MT, Brehme CS (2017) An improved camera trap for amphibians, reptiles, small mammals, and large invertebrates. PLoS ONE 1210:e0185026
Holmes RT (1986) Foraging patterns of forest birds: male-female differences. Wilson Bull 98(2):196–213
Hölzinger J (1994) Die Vögel Baden-Württembergs. Singvögel 1, vol 3.1. Ulmer, Stuttgart
Karwinkel T, Winklhofer M, Christoph P, Allenstein D, Hüppop O, Brust V, Schmaljohann H (2022) No apparent effect of a magnetic pulse on free-flight behaviour in northern wheatears (Oenanthe oenanthe) at a stopover site. J Royal Soc Interface 19:20210805
Kolowski JM, Forrester TD (2017) Camera trap placement and the potential for bias due to trails and other features. PLoS ONE 12(10):e0186679
Krauss SL, Roberts DG, Phillips RD, Edwards C (2018) Effectiveness of camera traps for quantifying daytime and nighttime visitation by vertebrate pollinators. Ecol Evol 818:9304–9314
Lehikoinen E (1987) Seasonality of the daily weight cycle in wintering passerines and its consequences. Ornis Scand 18:216–226
Luo G, Yang C, Zhou H, Seitz M, Wu Y, Ran J (2019) Habitat use and diel activity pattern of the Tibetan Snowcock (Tetraogallus tibetanus): a case study using camera traps for surveying high-elevation bird species. Avian Res 101:1–9
Main IG (2002) Seasonal movements of Fennoscandian blackbirds Turdus merula. Ring Migr 21:65–74
Meek PD, Ballard G, Claridge A, Kays R, Moseby K, O’Brien T, Townsend S (2014) Recommended guiding principles for reporting on camera trapping research. Biodiv Cons 23:2321–2343
Meredith M, Ridout M (2021) Package ‘Overlap’, version 0.3. 4
Møller AP, Jokimäki J, Skorka P, Tryjanowski P (2014) Loss of migration and urbanization in birds: a case study of the blackbird (Turdus merula). Oecologia 175:1019–1027
Orpwood JE, Griffiths SW, Armstrong JD (2006) Effects of food availability on temporal activity patterns and growth of Atlantic salmon. J Anim Ecol 753:677–685
Pereira JA (2010) Activity pattern of Geoffroy’s cats (Leopardus geoffroyi) during a period of food shortage. J Arid Environ 749:1106–1109
Pita R, Mira A, Beja P (2011) Circadian activity rhythms in relation to season, sex and interspecific interactions in two Mediterranean voles. Anim Behav 815:1023–1030
Post P, Götmark F (2006) Foraging behavior and predation risk in male and female Eurasian Blackbirds (Turdus merula) during the breeding season. Auk 1231:162–170
RStudio T (2020) RStudio: integrated development for R. Rstudio Team, PBC, Boston, MA URL http://www.rstudio.com
Randler C, Kalb N (2018) Distance and size matters: a comparison of six wildlife camera traps and their usefulness for wild birds. Ecol Evol 814:7151–7163
Randler C, Kalb J (2020) Predator avoidance behavior of nocturnal and diurnal rodents. Behav Processes 179:104214
Randler C, Pentzold S, Teichmann C (2010) Weather conditions and sexual differences affect the foraging behaviour of the insectivorous Cyprus Wheatear, Oenanthe cypriaca (Aves: Passeriformes: Muscicapidae). Vertebr Zool 60:175–181
Randler C, Pentzold S, Pentzold C (2015) Foraging behaviour of insectivorous migrants and a resident songbird at a stopover site. Biol 701:141–149
Randler C, Katzmaier T, Kalb J, Kalb N, Gottschalk TK (2020) Baiting/luring improves detection probability and species identification—a case study of mustelids with camera traps. Anim 1011:2178
Richardson E, Nimmo DG, Avitabile S, Tworkowski L, Watson SJ, Welbourne D, Leonard SW (2018) Camera traps and pitfalls: an evaluation of two methods for surveying reptiles in a semiarid ecosystem. Wildl 448:637–647
Ridout MS, Linkie M (2009) Estimating overlap of daily activity patterns from camera trap data. J Agricult Biol Environm Stats 14:322–337
Rogers CM, Reed AK (2003) Does avian winter fat storage integrate temperature and resource conditions? A long-term study. J Avian Biol 341:112–118
Rovero F, Zimmermann F, Berzi D, Meek P (2013) Which camera trap type and how many do I need? A review of camera features and study designs for a range of wildlife research applications. Hystrix 24(2):148–156
Rowcliffe JM (2023) Package activity. https://cran.r-project.org/web/packages/activity/activity.pdf
Rowcliffe JM, Kays R, Kranstauber B, Carbone C, Jansen PA (2014) Quantifying levels of animal activity using camera trap data. Methods Ecol Evol 511:1170–1179
Russ A, Rüger A, Klenke R (2015) Seize the night: European Blackbirds (Turdus merula) extend their foraging activity under artificial illumination. J Ornithol 156:123–131
Santiago-Quesada F, Masero JA, Estrella SM, Sánchez-Guzmán JM (2012) Persistent bimodal activity patterns in wild and captive black-tailed godwit Limosa limosa under different environmental conditions: a role for circadian rhythm? Behav Ecol Sociobiol 66:397–405
Schlicht L, Kempenaers B (2020) The effects of season, sex, age and weather on population-level variation in the timing of activity in Eurasian Blue Tits Cyanistes caeruleus. Ibis 1624:1146–1162
Steinmeyer C, Schielzeth H, Mueller JC, Kempenaers B (2010) Variation in sleep behaviour in free-living blue tits, Cyanistes caeruleus: effects of sex, age and environment. Anim Behav 805:853–864
Stuber EF, Dingemanse NJ, Kempenaers B, Mueller JC (2015) Sources of intraspecific variation in sleep behaviour of wild great tits. Anim Behav 106:201–221
Swanson DL, Olmstead KL (1999) Evidence for a proximate influence of winter temperature on metabolism in passerine birds. Physiol Biochem Zool 725:566–575
Van Vliet J, Musters CJM, Ter Keurs WJ (2009) Changes in migration behaviour of Blackbirds Turdus merula from the Netherlands. Bird Study 56:276–281
Vazquez C, Rowcliffe JM, Spoelstra K, Jansen PA (2019) Comparing diel activity patterns of wildlife across latitudes and seasons: time transformations using day length. Methods Ecol Evol 10:2057–2066
Wearn OR, Glover-Kapfer P (2019) Snap happy: camera traps are an effective sampling tool when compared with alternative methods. Royal Soc Open Sci 63:181748
Zimmermann F, Foresti D, Rovero F, Zimmermann F (2016) Capture-recapture methods for density estimation. In: Rovero F, Zimmermann F (eds) Camera trapping for wildlife research. Pelagic Publishing, Exeter, UK, pp 95–141
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Contributions
Conceptualization, CR, TKG; Methodology, CR, TKG, JD; Data collection (field), CR; Formal analysis, JD; Investigation, CR, XS; Data curation, JD, XS; Writing XS, NK, CR, TKG; All authors read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare no conflict of interest.
Animal ethics
This was an observational study and required no formal ethic application.
Additional information
Communicated by F. Bairlein.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Schlindwein, X., Randler, C., Kalb, N. et al. Seasonal variation in the diurnal activity pattern of Eurasian blackbirds (Turdus merula) in the forest. J Ornithol 165, 137–146 (2024). https://doi.org/10.1007/s10336-023-02096-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10336-023-02096-2