Abstract
In biparental Charadriinae plovers, male and female incubation duties often resemble daily routines, with males typically incubating at night and females incubating during the day. By analysing incubation behaviour in three Arctic populations of Common Ringed Plover Charadrius hiaticula, we show that these diel routines are lost in the 24-h sunlight of the Arctic. In contrast, a non-Arctic population in East Scotland exhibited significant daily routines, with males dominating incubation during the late afternoon and night, and females dominating incubation during the early morning and midday hours. These patterns suggest that clear light/dark cycles are necessary for daily incubation routines to form in the Common Ringed Plover, although further research is needed to understand the specific drivers of this behaviour.
Zusammenfassung
Brutverhalten des Sandregenpfeifers Charadrius hiaticula auf verschiedenen Breitengraden
Bei Charadriinae Regenpfeiferarten, bei denen sich beide Elternteile an der Brut beteiligen, folgen die Inkubationszeiten von Männchen und Weibchen oft einen Tagesablauf, wobei die Männchen in der Regel nachts und die Weibchen tagsüber brüten. Durch das Analysieren vom Brutverhalten in drei arktischen Populationen von Sandregenpfeifern Charadrius hiaticula konnten wir zeigen, dass diese täglichen Routinen im 24-Stunden-Sonnenlicht der Arktis verloren gehen. Im Vergleich dazu wies eine nichtarktische Population in Ostschottland ausgeprägte Tagesroutinen auf, wobei am späten Nachmittag und in der Nacht die Inkubation der Männchen und während des frühen Morgens und den Mittagsstunden die der Weibchen überwiegten. Diese Muster lassen vermuten, dass klare Hell-Dunkel-Zyklen für das Entstehen einer Brutroutine beim Sandregenpfeifer notwendig sind, auch wenn weitere Studien benötigt werden, um die genauen Ursachen dieses Verhaltens zu verstehen.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
During incubation, birds must balance the energetic costs and predation risk associated with nest attendance against the benefits of healthy egg development (Holt et al. 2002; Amat and Masero 2004; Smith et al. 2012; Bulla et al. 2016). In shorebirds, incubation responsibility is often shared between males and females, and the relative investment from each parent is expected to depend on the balance of such costs and benefits for each sex (Trivers 1972). Differences between males and females in their thermoregulatory abilities, energetic needs, crypsis and paternity/maternity confidence may all affect the relative investment of males and females (Trivers 1972; Wallander 2003; Bulla et al. 2014; Ekanayake et al. 2015). Understanding variation in parental cooperation during incubation is, therefore, a formidable task, but also an excellent opportunity to get a better understanding of the pressures birds face during reproduction.
Charadriinae plovers are a widespread subfamily of shorebirds, of which the majority are monogamous biparental incubators (Eberhart-Phillips 2019; Székely 2019). Previous research in many species from this clade has detected a greater male share of incubation at night than during the day (reported in the Snowy Plover Charadrius nivosus (Warriner et al. 1986; Kosztolányi and Székely 2002); Kentish Plover Charadrius alexandrinus (Fraga and Amat 1996; Vincze et al. 2013); Wilson’s Plover Charadrius wilsonia (Thibault and McNeil 1995); Killdeer Charadrius vociferus (Mundahl 1982; Warnock and Oring 1996); Two-banded Plover Charadrius falklandicus (St Clair et al. 2010a); Red-capped Plover Charadrius ruficapillus (Ekanayake et al. 2015); Semipalmated Plover Charadrius semipalmatus (Blanken and Nol 1998); White-fronted Plover Charadrius marginatus (Vincze et al. 2017); St. Helena Plover Charadrius sanctaehelenae (Burns et al. 2013); Hooded Dotterel Thinornis cuccullatus (Ryeland et al. 2022); Tibetan Sand Plover Charadrius atrifrons (Halimubieke et al. unpublished work); and Common Ringed Plover Charadrius hiaticula (Laven 1940; Wallander 2003). This effect also appears to be consistent between populations, with previous research showing evidence of a male bias towards night-time incubation in eight different temperate and tropical Kentish Plover populations (Vincze et al. 2013). This phenomenon has been suggested to reduce the risk of nest predation associated with incubation by more brightly coloured males (Ekanayake et al. 2015). However, some exceptions within the Charadriinae subfamily suggest the pattern is somewhat more complicated: the male bias towards night-time incubation persists in the sexually monomorphic St. Helena Plover and Hooded Dotterel (Burns et al. 2013; Ryeland et al. 2022), and the Rufous-chested Dotterel Charadrius modestus displays the opposite pattern of a female bias towards incubation at night-time, despite more highly contrasting males (St Clair et al. 2010b). Alternatively, the high physiological effort of egg production for females may underlie the sex difference in incubation behaviour (female Common Ringed Plovers can produce up to five clutches in a single season, containing ~ 3.7 times the female body mass (Wallander & Andersson 2003)). Such costs may leave females less able to pay the higher thermoregulation costs of incubation in cold conditions (Vleck 1981; Skutch 1957), and/or may require females to exploit the optimal foraging conditions at night (Blanken and Nol 1998; Robert and McNeil 1989; Kuwae 2007).
The Common Ringed Plover (hereafter Ringed Plover) breeds in a particularly wide spread of latitudes, including temperate regions with clear day/night cycles, and Arctic regions with continuous summer daylight, providing an opportunity to test whether continuous daylight removes the male bias in night-time incubation behaviour. Previous research in this species has indicated that incubation patterns may indeed vary by latitude. Two previous studies in temperate regions along the Baltic Sea identified a male bias in night-time incubation (Laven 1940; Wallander 2003), whilst a single study in Arctic Greenland did not (Pienkowski 1984). However, these results were not clear-cut, with the pattern of incubation in one temperate population based on few night-time nest visits (Laven 1940), and the trend towards male-biased night-time incubation in the other temperate population falling short of statistical significance (Wallander 2003). Here, we aim to clarify the effect of latitude on incubation behaviour in the Ringed Plover, via continuous recording of nests in four populations that reflect most of the latitudinal range of the species. This question will provide a clue as to the drivers of incubation behaviour in Charadriinae plovers: theories focussing on the role of thermoregulation may predict a continued benefit to male-biased night-time incubation in the Arctic, given that temperature cycles drive greater nest attendance at night in Arctic-breeding uniparental shorebirds (Tulp and Schekkerman 2006; Steiger et al. 2013; Bulla et al. 2014). On the other hand, theories focussing on the role of predators relying on visual cues, or those focussing on night-time foraging efficiency, may predict little benefit to male-biased night-time incubation under the midnight sun (Eriksen and Wabakken 2018).
Methods
Populations
Fieldwork was conducted in four Ringed Plover populations between 2019 and 2022 (Fig. 1). Nests from the ‘Angus’ population are located on or near the east coast of Scotland, with northern and southern limits in the towns of Edzell (56° 49ʹ N, 2° 39ʹ W) and Collessie (56° 19ʹ N, 3° 9ʹ W). Nest cameras were placed between 15 May 2021 and 21 June 2021. The Angus population likely consists of the subspecies Charadrius hiaticula hiaticula (Engelmoer and Roselaar 2012). The ‘Tobseda’ population is located on the Arctic coast of the Nenets region of European Russia within 5 km of the abandoned settlement of Tobseda (68° 35ʹ N, 52° 18ʹ E), and nest cameras were placed between 29 June 2019 and 16 July 2019. The Tobseda population consists of the subspecies C. h. tundrae (Thies et al. 2018). The ‘Varanger’ population is located on the East coast of the Varanger peninsula of Norway, with northern and southern limits of Persfjorden (70° 26ʹ N, 30°47ʹ E) and Skallelv (70° 11ʹ N, 30° 20ʹ E). Here, nest cameras were placed between 05 June 2022 and 24 June 2022. This area represents an admixture zone of two subspecies: C. h. tundrae and C. h. hiaticula (Thies et al. 2018). The ‘Ny-Ålesund’ nests were located within 5 km of the village of Ny-Ålesund in Svalbard (78° 55ʹ N, 11° 55ʹ E), and nest cameras were placed between 14 July 2022 and 16 July 2022. The subspecies of this population is unclear (Tomkovich and Serra 1999; Engelmoer and Roselaar 2012).
Location of the studied populations (labelled black dots) within the Ringed Plover breeding range (turquoise; visible as a lighter grey in black and white images). Breeding range includes resident populations and is based on data from BirdLife International (2022)
Incubation data
Ringed Plover nests consist of a shallow scrape on the ground, lined with varying amounts of substrate (e.g. lichen, pebbles and shells). Clutch size is typically 3–4 eggs, and both sexes incubate for ca. 25 days following clutch completion (Pienkowski 1984). Nests were found either by retreating from and observing alarming Ringed Plovers, or by searching suitable nesting habitats. For the Tobseda, Varanger and Ny-Ålesund populations, the approximate nest age was estimated by egg floatation or observation during the laying period (Liebezeit et al. 2007). For the Angus population, eggs were not floated, but nest age could be estimated for 7/9 nests based on observed laying and hatching dates. Clutch sizes for observed nests varied from 3 to 5 eggs, and all observations were undertaken following clutch completion.
For nest monitoring, we used small security cameras with infrared LEDs (‘Wyzecams’ or ‘Neos Smartcams’), which were connected to 15,000 mAh or 30,000 mAh power banks, and waterproofed by placing within plastic bottles covered in camouflaging tape. These cameras were placed ~ 50 cm from a nest and recorded continuously for 24–72 h. The observation period began at least 1 h after camera placement to allow normal incubation behaviour to resume following the minor disturbance. One hour was generally more than sufficient (incubation tended to resume within 10 min); however, if the birds had not yet returned after 1 h (4/46 observations), the observation only began once they returned. When nests were disturbed by the capture of one or more parents, at least 24 h was left until observations began. Footage was analysed in 24-h or 48-h ‘observations’ using BORIS software (Friard and Gamba 2016), with the data exported in 24-h portions and processed using a custom R code (R core team 2020).
The sex of the incubating individual was identified primarily by head colouration, which is more contrasting in males than females (Meissner et al. 2010). For 20 out of 32 nests, at least 1 incubating adult was colour ringed at the time of observation, and these rings were occasionally used to separate the sexes when they swapped without facing the nest camera. For these 20 nests (4 from Angus, 7 from Tobseda, 6 from Varanger, and 3 from Ny-Ålesund), feather or blood sampling allowed visual sexing to be confirmed using molecular methods. In all 20 cases, the visually assigned sex was correct, confirming both that the sexes can be separated visually by head colouration, and that males consistently exhibit more contrasting colouration than females in these populations (at least from a human observer’s perspective). 24-h periods where the incubating parent could not be clearly identified for more than 1% of the time were excluded (4 out of 66 24-h periods). In total, about 1500 h of observation from 32 nests were included in the final analysis. These observations were split across the four populations as follows: 5 × 24-h and 4 × 48-h observations in Angus across 9 nests, 18 × 24-h observations in Tobseda across 9 nests, 11 × 48-h observations and 5 × 24-h observations in Varanger across 11 nests, 2 × 24-h observations and 1 × 48-h observation in Ny-Ålesund across 3 nests. Note that due to the small sample size, results concerning the Ny-Ålesund population should be treated with caution. Nevertheless, the data from these three nests remain pertinent to the larger question as to whether Ringed Plover incubation routines are present in Arctic populations.
Molecular sexing
Molecular sexing was achieved through PCR amplification of the Chromo-Helicase-DNA binding protein gene (CHD), using 2602F and 2669R primers (van der Velde et al. 2017). DNA was extracted from feather samples via ammonium acetate precipitation (Bruford et al. 1998), and DNA was extracted from blood samples via either ammonium acetate precipitation or alkaline lysis (Bruford et al. 1998; Rudbeck and Dissing 1998). Feather samples were plucked from the flanks under the closed wing, and blood samples were collected from the brachial vein (Székely et al. 2008).
Temperature and sun altitude data
To understand whether incubation routines were more closely associated with light or temperature cycles, data on sun altitude and air temperature were collated. In Tobseda, rolling average air temperature was recorded every 4 h using a probe ~ 500 m from the midpoint of the population. For the other three populations, temperature data were collected from the closest meteorological station with the relevant hourly records: Leuchars station (56° 22ʹ N, 2° 52ʹ W, approx. 33 km south of the midpoint of the Angus population); Vardø Airport station (70° 21 N, 31° 3 E, approx. 8 km east of the midpoint of the Varanger population); Svalbard Airport station (78° 15ʹ N, 15° 28 E, approx. 107 km south-east of the midpoint of the Ny-Ålesund population). Sun altitude data were collected for each population using the ‘suncalc’ R package (Thieurmel 2019).
Statistical analysis
Two dependent variables were used for all analyses—the proportion of time the nest was attended by either the male or the female (‘nest attendance’), and the proportion of incubation that was performed by males (‘male share’). Male share was normally distributed and was investigated using the raw values. Raw values for nest attendance were highly skewed and this variable was analysed following a natural log transformation of nest absence (Ln(1-attendance)). Also included in the dataset: nest ID (unique for each nest), time of day (either ‘day’, the 8 h where sun altitude was highest, or ‘night’, the 8 h where sun altitude was lowest, defined to encompass the length of the longest night of observation in Angus (sunset 21.28 h, sunrise 04.49 h)), nest age (days since clutch completion) and population (Angus, Tobseda, Varanger or Ny-Ålesund). Note that the length of ‘day’ and ‘night’ (i.e. 8 h) were matched both to meet the statistical assumption of equal variance amongst groups, and to ensure statistical power was not weakened by unclear predictions regarding behaviour in the hours shortly before sunset and shortly after sunrise. The sun did not set during any observations of the three Arctic populations (Tobseda, Varanger and Ny-Ålesund).
Statistical analysis was conducted in R version 4.0.1 (R Core Team 2020), and model assumptions were assessed visually. The main analysis used linear mixed effect models to test whether the effect of time of day on nest attendance and the male share of incubation was consistent amongst populations (using the ‘lmer’ function in the ‘lme4’ package; Bates 2010). Observations across multiple 24-h periods were averaged so that there was a single value for male share and nest attendance for ‘night’ and for ‘day’ for each nest. A secondary analysis concerned overall patterns in the dataset across the different populations, and values for ‘male share’ and ‘nest attendance’ were averaged across observations and across all 24 h to produce single value per nest. This secondary analysis tested for a relationship between male share and nest attendance (using a general linear model), and whether the male share was significantly different from 0.5 (using a 1-sample t-test). There was no difference in the mean age of nests between populations (ANOVA p = 0.27), and an initial analysis found no impact of nest age on male share or total nest attendance (results not shown), so nest age was excluded from models to avoid over-parameterisation. However, visual inspection of the data revealed that the Angus population showed a particularly high proportion of observations of very young nests (4/9 nests < 5 days old), and so patterns in this population were checked for consistency across young and old nests.
Data availability
The final dataset and analysis code have been deposited onto Zenodo (https://doi.org/10.5281/zenodo.7614926), along with the custom code used to transform the BORIS data output, and some example clips from nest cameras. Raw data exports from BORIS, as well as nest camera videos, are available upon request.
Results
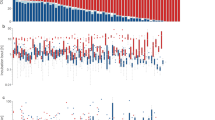
The effect of time of day on the male share of incubation was significantly different between populations (Fig. 2, Table 1). Paired t tests compared day-time and night-time incubation patterns within each population, and revealed that the male share of incubation was significantly greater at night than during the day in the temperate Angus population (t = 2.77, n = 9, p = 0.024; mean day-time share = 0.42 (95% CI = 0.33–0.51), mean night-time share = 0.60 (95% CI = 0.47–0.74)). In contrast, no effect of time of day was apparent in Tobseda (t = 0.86, n = 9, p = 0.42), Varanger (t = 0.31, n = 11, p = 0.76) or Ny-Ålesund (t = 0.49, n = 3, p = 0.67). To check whether the over-representation of very young nests in the Angus population was driving this effect, mean male share at day and night in young (< 5 days) Angus nests (n = 4) was compared with old (> 18 days) Angus nests (n = 3). The two sets of nests showed very similar patterns (young nest day-time male share = 0.39, old nest day-time male share = 0.40; young nest night-time male share = 0.62, old nest night-time male share = 0.66), making it unlikely that the over-representation of very young nests is driving the difference between populations. In the Angus population, temperatures were significantly warmer in the 2 h prior to sunset (19:00–21:00), than in the 2 h following sunrise (05:00–07:00), due to heat loss throughout the night (paired t test: t = 5.19, n = 9, p < 0.001). This was exploited to test whether temperature was the sole driver of the male bias towards night-time incubation, by testing whether males also incubated more during cold conditions when the sun was up. A paired t test revealed the opposite pattern: the male share of incubation was significantly lower in the 2 h following sunrise than in the 2 h prior to sunset (t = 2.88, n = 9, p = 0.021; mean male share 05:00–07:00 = 0.32 (95% CI = 0.19–0.46); mean male share 19:00–21:00 = 0.63 (95% CI 0.35–0.89)).
Male share of incubation in relation to time of day, in four populations of Common Ringed Plover (Angus (top left), Tobseda (top right), Varanger (bottom left) and Ny-Ålesund (bottom right)). Larger plots show the average male share of incubation for each nest split by ‘day’ and ‘night’. Inset bar plots show the average male share of incubation for each population over 24 h, with lines showing average temperature (dashed line) and sun altitude (solid line) curves during nest observations. A horizontal line shows the altitude of the horizon (labelled ‘Sunrise/Sunset’). In the temperate Angus population, Common Ringed Plover males incubate more at night than during the day, and show a clear routine over the 24-h period, whereas no such pattern is present for the three Arctic populations. p values reflect paired t tests for each population (day-time male share vs. night-time male share)
The effect of time of day on nest attendance was also significantly different between populations (Fig. 3, Table 2). Paired t tests comparing day-time and night-time nest attendance in each population revealed a significantly higher night-time than day-time nest attendance in Tobseda (t = 3.00, n = 9, p = 0.017; back-transformed mean day-time nest attendance = 0.955 (95% CI = 0.907–0.978), back-transformed mean night-time nest attendance = 0.984 (95% CI = 0.956–0.994)). In contrast, no effect of time of day was found in Angus (t = − 1. 04, n = 9, p = 0.33), Varanger (t = 0.41, n = 11, p = 0.69) or Ny-Ålesund (t = 1.61, n = 3, p = 0.25). This may reflect the more extreme daily temperature variation in Tobseda compared with the other sites (see inset temperature curves of Fig. 3). Analysis of trends across the combined populations found a tendency for males to incubate less than females, although this difference was not statistically significant (p = 0.064, summary data shown in Supplementary Table 1), and was unrelated to total nest attendance (p = 0.657).
Total nest attendance in relation to time of day, in four populations of Common Ringed Plovers (Angus (top left), Tobseda (top right), Varanger (bottom left) and Ny-Ålesund (bottom right)). Larger plots show the average nest attendance for each nest split by ‘day’ and ‘night’. Inset bar plots show the average nest attendance for each population over 24 h, with lines showing average temperature (dashed line) and sun altitude (solid line) curves during nest observations. A horizontal line shows the altitude of the horizon (labelled ‘Sunrise/Sunset’). Note that the scale of the plot is different for the Angus population. In Tobseda, nest attendance is significantly higher at night than during the day, whereas no such effect is present for the other three populations. p values reflect paired t tests for each population (day-time attendance vs. night-time attendance)
Discussion
Across all four Ringed Plover populations, incubation was approximately evenly split between males and females, which is not unusual amongst Charadriinae plovers (Eberhart-Phillips 2019), and is consistent with previous research in the Ringed Plover (Pienkowski 1984; Wallander 2003). The frequently found bias towards night-time incubation in male Charadriinae plovers was present in a temperate population of the Ringed Plover, located in Angus, East Scotland (Fig. 2). The strength of the male bias towards night-time incubation in the Angus population (60% of night-time incubation vs. 42% of day-time incubation) was similar to that reported in Ringed Plovers in southern Sweden (58% night-time incubation vs. 45% day-time incubation), although this did not reach statistical significance in the previous study (Wallander 2003). This is a much weaker bias than has been described in some Charadrius species, where male and female incubation duties are almost entirely split according to time of day, e.g. the Red-capped Plover and Two-banded Plover (Ekanayake et al. 2015; St Clair et al. 2010a). These species exhibit similar levels of sexual dimorphism to the Ringed Plover (Székely et al. 2022), and the difference in behaviour may instead reflect their breeding latitude. St. Clair et al. (2010a) highlight that specialised roles in incubation may lead to increasing inequality in male and female reproductive effort for populations close to the poles, as days and nights become unequal in length. The relatively long summer days in northern temperate regions may, therefore, explain the lack of rigidity in Ringed Plover diel patterns, as strict day/night specialisation of duties could constitute an unsustainable loss of foraging opportunity for females.
In contrast to the temperate Angus population, the male share of incubation did not differ between day and night in our three Arctic Ringed Plover populations. This might be explained by the 24-h sunlight in Artic regions during the nesting period and is consistent with a previous study of Ringed Plovers in Greenland (Pienkowski 1984). Yet, it is not the rule for all Arctic-breeding shorebirds, where daily roles in male and female incubation behaviour have been detected despite 24-h sunlight (Bulla et al. 2014). In the summer months, temperature generally shows reduced daily variation in Arctic regions compared to temperate and tropical regions, and this effect was visible in the daily temperature curves of the two highest latitude sites (Varanger and Ny-Ålesund, see inset plots of Fig. 2 or Fig. 3). In contrast, the Arctic Tobseda site had slightly higher levels of daily temperature variation than the temperate Angus site, and was the only population where overall nest attendance was greater at night (Fig. 3). The absence of a male bias towards night-time incubation in the Tobseda population, therefore, suggests that temperature variation alone does not drive the male bias towards night-time incubation. Furthermore, males from the Angus population provided a greater share of incubation during the warm hours leading up to sunset, compared with the cold hours immediately following sunrise, which is also inconsistent with temperature being the sole driver of incubation routines. This tendency for males to incubate during the warmer part of the day, as well as during the cold night, may reflect a compromise between the cost of thermoregulation for incubating males, and a light-dependent selection pressure (e.g. reducing nest predation by visual predators, or allowing females to forage during the dark hours). Further research is required to fully understand the selection pressures underlying the incubation behaviour of Charadriinae plovers, and variation amongst Ringed Plover populations provides a useful background for such studies. Comparing the thermoregulation costs of incubation between males and females may be particularly interesting, as the current results suggest that males in temperate populations pay a higher proportion of thermoregulation costs than males in the Arctic.
References
Amat JA, Maseru JA (2004) Predation risk on incubating adults constrains the choice of thermally favourable nest sites in a plover. Anim Behav 67:293–300
Bates DM (2010) lme4: Mixed-effects modeling with R
BirdLife International and Handbook of the Birds of the World (2022) Bird species distribution maps of the world. Version 2022.2
Blanken MS, Nol E (1998) Factors affecting parental behavior in Semipalmated Plovers. Auk 115:166–174
Bruford MW, Hanotte O, Brookfield JFY, Burke T (1998) Multilocus and single-locus DNA fingerprinting. Mol Genet Anal Popul 2:287–336
Bulla M, Valcu M, Rutten AL, Kempenaers B (2014) Biparental incubation patterns in a high-Arctic breeding shorebird: how do pairs divide their duties? Behav Ecol 25:152–164
Bulla M, Valcu M, Dokter AM et al (2016) Unexpected diversity in socially synchronized rhythms of shorebirds. Nature 540:109–113
Burns F, McCulloch N, dos Remedios N et al (2013) Sex differences in incubation behaviour but not mortality risk in a threatened shorebird. Ibis 155:877–880
Eberhart-Phillips LJ (2019) Plover breeding systems: diversity and evolutionary origins. The population ecology and conservation of Charadrius plovers. CRC Press, Boca Raton, pp 65–88
Ekanayake KB, Weston MA, Nimmo DG et al (2015) The bright incubate at night: sexual dichromatism and adaptive incubation division in an open-nesting shorebird. Proc R Soc B 282:20143026
Engelmoer M, Roselaar CS (2012) Geographical variation in waders. Springer Science & Business Media, Berlin
Eriksen A, Wabakken P (2018) Activity patterns at the Arctic Circle: nocturnal eagle owls and interspecific interactions during continuous midsummer daylight. J Avian Biol 49:e01781
Fraga RM, Amat JA (1996) Breeding biology of a kentish plover (Charadrius alexandrinus) population in an inland saline lake. Ardeola 43:69–85
Friard O, Gamba M (2016) BORIS: a free, versatile open-source event-logging software for video/audio coding and live observations. Methods Ecol Evol 7:1325–1330
Holt S, Whitfield DP, Duncan K et al (2002) Mass loss in incubating Eurasian dotterel: adaptation or constraint? J Avian Biol 33:219–224
Kosztolányi A, Székely T (2002) Using a transponder system to monitor incubation routines of snowy plovers. J Field Ornithol 73:199–205
Kuwae T (2007) Diurnal and nocturnal feeding rate in Kentish plovers Charadrius alexandrinus on an intertidal flat as recorded by telescopic video systems. Mar Biol 151:663–673
Laven H (1940) Beiträge zur Biologie des Sandregenpfeifers (Charadrius hiaticula L.). J Ornithol 88:183–287
Liebezeit JR, Smith PA, Lanctot RB et al (2007) Assessing the development of shorebird eggs using the flotation method: species-specific and generalized regression models. Condor 109:32–47
Meissner W, Chylarecki P, Skakuj M (2010) Ageing and sexing the Ringed Plover Charadrius hiaticula. Wader Study Group Bulletin 117:99–102
Mundahl JT (1982) Role specialization in the parental and territorial behavior of the Killdeer. Wilson Bull 94:515–530
Pienkowski MW (1984) Breeding biology and population dynamics of Ringed plovers Charadrius hiaticula in Britain and Greenland: nest-predation as a possible factor limiting distribution and timing of breeding. J Zool 202:83–114
Robert M, McNeil R (1989) Comparative day and night feeding strategies of shorebird species in a tropical environment. Ibis 131:69–79
Rudbeck L, Dissing J (1998) Rapid, simple alkaline extraction of human genomic DNA from whole blood, buccal epithelial cells, semen and forensic stains for PCR. Biotechniques 25:588–592
Ryeland J, Magrath MJL, Weston MA (2022) Day–night cycle influences the division of incubation in the Hooded Dotterel (Thinornis cucullatus). Ibis 164:785–792
Skutch AF (1957) The incubation patterns of birds. Ibis 99:69–93
Smith PA, Tulp I, Schekkerman H et al (2012) Shorebird incubation behaviour and its influence on the risk of nest predation. Anim Behav 84:835–842
St Clair JJH, Herrmann P, Woods RW, Székely T (2010a) Female-biased incubation and strong diel sex-roles in the Two-banded Plover Charadrius falklandicus. J Ornithol 151:811–816
St Clair JJH, Kuepper C, Herrmann P et al (2010b) Unusual incubation sex-roles in the Rufous-chested Dotterel Charadrius modestus. Ibis 152:402–404
Steiger SS, Valcu M, Spoelstra K et al (2013) When the sun never sets: diverse activity rhythms under continuous daylight in free-living arctic-breeding birds. Proc Royal Soc B 280:20131016
Székely T (2019) Why study plovers? The significance of non-model organisms in avian ecology, behaviour and evolution. J Ornithol 160:923–933
Székely T, Kosztolányi A, Küpper C (2008) Practical guide for investigating breeding ecology of Kentish plover. Charadrius alexandrinus. Unpublished report University of Bath, Bath
Székely T, Liker A, Thomas GH, et al (2022) Sex roles in birds: influence of climate, life histories and social environment. Dryad DatasetTeam RC, others (2020) R: A language and environment for statistical computing. R Foundation for Statistical Computing
Thibault M, McNeil R (1995) Day-and night-time parental investment by incubating Wilson’s Plovers in a tropical environment. Can J Zool 73:879–886
Thies L, Tomkovich P, dos Remedios N et al (2018) Population and subspecies differentiation in a high latitude breeding wader, the Common Ringed Plover Charadrius hiaticula. Ardea 106:163–176
Thieurmel B, Elmarhraoui A, Thieurmel MB (2019) Package ‘suncalc’. R package version 0.5
Tomkovich PS, Serra L (1999) Morphometrics and prediction of breeding origin in some Holarctic waders. Ardea 87:289–300
Trivers R (1972) Parental investment and sexual selection. U: Sexual Selection & the Descent of Man. Campbell, BG: 136–179
Tulp I, Schekkerman H (2006) Time allocation between feeding and incubation in uniparental arctic-breeding shorebirds: energy reserves provide leeway in a tight schedule. J Avian Biol 37:207–218
van der Velde M, Haddrath O, Verkuil YI et al (2017) New primers for molecular sex identification of waders. Wader Study 124:147–151
Vincze O, Szekely T, Küpper C et al (2013) Local environment but not genetic differentiation influences biparental care in ten plover populations. PLoS ONE 8:e60998
Vincze O, Kosztolányi A, Barta Z et al (2017) Parental cooperation in a changing climate: fluctuating environments predict shifts in care division. Glob Ecol Biogeogr 26:347–358
Vleck CM (1981) Energetic cost of incubation in the zebra finch. Condor 83:229–237
Wallander J (2003) Sex roles during incubation in the Common Ringed Plover. Condor 105:378–381
Wallander J, Andersson M (2003) Reproductive tactics of the ringed plover Charadrius hiaticula. J Avian Biol 34:259–266
Warnock N, Oring LW (1996) Nocturnal nest attendance of Killdeers: more than meets the eye. Auk 113:502–504
Warriner JS, Warriner JC, Page GW, Stenzel LE (1986) Mating system and reproductive success of a small population of polygamous Snowy Plovers. Wilson Bull 98:15–37
Acknowledgements
Many thanks to the many helpful researchers who contributed by finding nests and helping with sample collection during field seasons, especially Chiel Boom, Ingrid Pollet, Mo Verhoeven, Jesper Mosbacher, Thomas Lameris, Freya Coursey and Rachael Wilbourn. Thanks also to Mike Bruford and Rachael Wilbourn (again) for their DNA extractions advice, and to Nolwenn Fresneau and Zsófia Tóth for their help with initial processing and storage of the nest camera videos. This work was supported by the National Environmental Research Council (NE/S007504/1 to KW), the Svalbard Science Forum (Arctic Field Grant project 322576 to GWG and KW), the Taif University Researchers Supporting Project (TURSP-2020/225 to MA), the Polar Programme of Netherlands Organisation for Scientific Research (ALWPP.2016.030 to GE), the Statoil Research fund to OH and TL, the Royal Society (WM170050 and APX\R1\191045 to TS), the National Research, Development and Innovation Office of Hungary (KKP-126949 to TS), and a University of Bath Developing Networks in Europe Grant to TS. All required permissions were granted by the relevant authorities in each country, and the sampling was completed as part of a project approved by the University of Bath’s Animal Welfare and Ethical Review Body.
Author information
Authors and Affiliations
Contributions
KW and TS conceptualised the study. KW, OH, TL, CM, GE and GWG organised fieldwork, gathered nest camera recordings and collected feather/blood samples. MA, KW, VA and LK coded the nest camera recordings. KW processed the data, performed molecular sexing, completed the statistical analysis, created the figures, and wrote the manuscript. All authors discussed results and commented on the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no competing interests to declare.
Additional information
Communicated by F. Bairlein.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wanders, K., Almalki, M., Heggøy, O. et al. Incubation behaviour of the Common Ringed Plover Charadrius hiaticula at different latitudes. J Ornithol 164, 825–833 (2023). https://doi.org/10.1007/s10336-023-02077-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10336-023-02077-5