Abstract
Our current understanding of the function of coordinated acoustic displays usually comes from studies conducted over a short period of the breeding season. However, the function of particular types of vocalizations may vary according to sex and context, and such displays can extend beyond the time of reproduction. To fully understand this phenomenon, analyses of year-round singing behavior are required. In the current study, we focused on a small, year-round territorial Afrotropical songbird, Chubb’s Cisticola (Cisticola chubbi). We analyzed the structure of songs during the breeding season as well as year-round changes in the proportion of solos, duets, and choruses to investigate the potential function(s) of each type of vocalization. We found that: (1) females produced whistling notes, while males generated trilling ones; (2) up to five individuals formed coordinated choruses, and (3) individuals were always near to each other during cooperative singing. Over the course of a year, the majority of syllables recorded were duets (82%), with rarer choruses (16%) and extremely rare solos (2%). Outside of the breeding season, males produced the most solos, while females produced more at the beginning of the breeding season. The proportion of choruses was highest at the end of breeding season. Frequent year-round production of duets and choruses strongly supports territory defense as the main function of joint singing, while the highest proportion of choruses at the end of the breeding season suggests that offspring take part in the chorus. To better understand cooperative singing, it is essential to extend our looking beyond the breeding season.
Zusammenfassung
Häufige Duette, seltene Chöre und extrem seltene Solos – das ganzjährige Singverhalten des Farnzistensängers.
Unser derzeitiger Wissensstand zur Funktion koordinierter akustischer Darbietungen stammt in der Regel aus Studien, die über einen kurzen Zeitraum während der Brutsaison durchgeführt wurden. Aber die Funktion bestimmter Arten von Lautäußerungen kann je nach Geschlecht und Kontext variieren, und diese Lautäußerungen können über die Zeit der Fortpflanzung hinaus andauern. Um dieses Verhalten vollständig zu verstehen, braucht man Analysen des ganzjährigen Gesangsverhaltens. In dieser Untersuchung konzentrierten wir uns auf einen kleinen, ganzjährig territorialen afrotropischen Singvogel, den Farnzistensänger (Cisticola chubbi). Wir analysierten die Struktur des Gesangs während der Brutzeit sowie die ganzjährigen Veränderungen des Anteils von Solos, Duetten und Chören, um die potenziellen Funktion(en) der einzelnen Gesangsarten zu untersuchen. Dabei stellten wir fest, dass (1) die Weibchen pfeifende Töne erzeugen, während die Männchen trillernde Töne von sich geben; (2) bis zu fünf Individuen koordinierte Chöre bilden und (3) die Individuen während des gemeinsamen Gesangs immer nahe beieinander sind. Im Laufe eines Jahres konnten wir die meisten Silben in den Duetten aufnehmen (82%), seltener im Chor (16%) und äußerst selten in Solos (2%). Außerhalb der Brutzeit produzierten die Männchen die meisten Sologesänge, während die Weibchen mehr Solos zu Beginn der Brutzeit von sich gaben. Der Anteil der Chöre war zum Ende der Brutzeit am höchsten. Die häufige, ganzjährige Produktion von Duetten und Chören spricht stark für die Revierverteidigung als Hauptfunktion des gemeinsamen Gesangs, während der höchste Anteil an Chören am Ende der Brutsaison darauf hindeutet, dass der Nachwuchs dann in den Chören mitsingt. Um das gemeinsame Singen besser zu verstehen, muss der Blick über die Brutzeit hinausgehen.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Acoustic communication has been intensively studied in birds, but mainly in temperate regions. Therefore, initially, bird song was defined as a complex vocalization produced by males in the breeding season to attract females and repel rivals (Catchpole and Slater 2008). However, more recent studies have shown that (1) female singing is quite widespread in birds, especially in the tropics, and may be observed in approximately two-thirds of songbirds (Odom et al. 2014), (2) the fact that females of temperate species are rarely observed singing is not the effect of an increase in singing by males but of a rapid loss of singing abilities in females (Price et al. 2009), and (3) sedentary tropical species sing all year round (Vokurková et al. 2018). Furthermore, in many bird species, males and females coordinate their vocalizations to produce duets and choruses (Soma and Brumm 2020; Hall 2009).
Collective singing is estimated to occur in 18% of bird species (Tobias et al. 2016), and evolutionarily speaking, is thought to be promoted by year-round territoriality and group living (Tobias et al. 2016; Soma and Brumm 2020), but not necessarily by sexual monochromatism or tropical breeding (Logue and Hall 2014). Recent research suggests that collective singing has evolved independently in several different groups of birds (Soma and Brumm 2020). According to current theory, (1) duets and choruses characterized by similar acoustic structure in males and females may be an ancestral state; (2) the vocal complexity of cooperative acoustic display may indicate social complexity, (3) extensive sex-based differences in acoustic structure in cooperatively signaling species may be the effect of selection for or against complex signals in one sex; and (4) cooperative acoustic signaling may have first evolved before, and independently of, female song (Hall 2009; Leighton 2017).
Vocal duets have been described in ca. 40% of bird families, although this number is still growing (Hall 2004, 2009; Tobias et al. 2016). Duets are mostly produced between a male and a female (reviewed in Hall 2009), but singing by two males has also been observed (e.g., DuVal 2007). Duets are predominantly created by a female responding to a male’s song (e.g., Mennill and Vehrencamp 2005; Klenova et al. 2008; Koloff and Mennill 2012), but in some species and contexts, males may also create duets by responding to a female’s song (e.g., Dahlin and Benedict 2014). The phrases, syllables, or notes sung by the two individuals may be sex-specific (e.g., Wright and Dahlin 2007; Templeton et al. 2015) or indistinguishable between the sexes (e.g., Mennill and Vehrencamp 2005; Benedict and McEntee 2009; Koloff and Mennill 2012). Individuals may sing antiphonally as well as synchronously (e.g., Grafe and Bitz 2004; Wright and Dahlin 2007) and be located either near or far away from each other (e.g., Mennill and Vehrencamp 2008). The extent of variation in duet structure both within and among species has raised doubts regarding its function (Hall 2009; Templeton et al. 2015). However, recent work has suggested that the evolution of duets is strongly associated with the coordinated year-round defense of ecological resources by both males and females, while other explanations of duetting seem to be by-products of this underlying trend (Tobias et al. 2016). Thus, the different proposed functions of duets (e.g., mate or paternity guarding, signaling the quality of a pair, signaling commitment to the partner, maintaining contact, individual recognition, or reproductive synchrony) are not mutually exclusive. Indeed, the underlying function may vary both among and within species because of changes in the motivation toward resource defense over time (Hall 2004; Dahlin and Benedict 2014).
Compared with duets, choruses are less common and correspondingly less studied. By definition, a chorus is much more complex than a duet, since more than two individuals are involved in singing together. In some species females and males sing alternating phrases in chorus, but with two or more members of the same sex producing the same type of phrase at the same time; the result sounds (and looks on spectrograms) like an echo (e.g., Mann et al. 2006). In another species individuals taking part in a chorus may produce overlapping syllables with low level of coordination or alternating and precisely coordinated ones, when both individuals of the same as well as opposite sex sing in alternating way (e.g., Seddon 2002). The primary function of choruses seems to be joint territorial defense (Baker 2004; Baker 2009; Bradley and Mennill 2009; Tobias et al. 2016). However, choruses, like duets, may have multiple functions, including coordination of group activity; maintaining contact; encoding group-specific identity; or signaling the number of individuals in, commitment to, or social structure of a group (Seddon and Tobias 2003; Baker 2004; Hale 2006; Bradley and Mennill 2009).
To fully understand the evolution and function of duets and choruses, it is first necessary to have reliable information on the most basic characteristics of these coordinated vocalizations, such as the acoustic structure of vocal elements produced by individuals, or the prevalence rate of solos, duets, and choruses, across a wide range of bird taxa (Bradley and Mennill 2009). Without this knowledge, it will be challenging to gain a true understanding of the functions of collective singing. Moreover, our current understanding of the function of birdsong, including duets and choruses, comes in large part from studies conducted in strongly seasonal environments, in which the breeding season is short and highly synchronized at the population level. Even in studies of sedentary species, researchers usually focus on a very short period of the breeding season (but see Odom et al. 2017; Voigt et al. 2021). However, vocal signaling behavior can vary considerably over both short and long periods and strongly depends on the breeding stage of communicating individuals (Bradley and Mennill 2009; Diniz et al. 2018; Vokurková et al. 2018). Thus, it is important to examine changes in vocal signaling throughout the year in order to fully understand the overall variation in and potentially changing functions of different types of vocalizations, such as solos, duets, and choruses, over time (Odom et al. 2017; Voigt et al. 2006). Furthermore, in environments that are stable year-round, breeding cycles may be less synchronized at the population level (e.g., Moore et al. 2005), and a variety of signaling behavior may be observed at the same time.
In the study, we focused on year-round cooperative singing behavior in a small Afrotropical songbird species without sexual dimorphism—the Chubb’s Cisticola (Cisticola chubbi) (Passeriformes, Cisticolidae). The genus Cisticola contain 51 species inhabiting grasslands, savannahs, marshes, and woodlands, primarily in Africa (Winkler et al. 2020). While most of the species form monogamous pairs, some are polygynous or breed cooperatively. Depending on the species, both partners or the female alone may build the nest, incubate, and feed nestlings. Females lay between one and seven eggs, and the nestlings leave the nest after 13–16 days. The generation time in cisticolas is one of the shortest known in birds, with some individuals breeding just 27 days after fledging (Winkler et al. 2020). In most cisticolas mainly the males sing. Duets have been found in six species, and in some of them, more than two individuals sing together forming choruses. Flicking wings and tail during a singing has been observed in some cisticolas, suggesting multimodal signaling. Yet, neither multimodal signaling nor acoustic communication have been closely studied in cisticolas in the past.
The Chubb’s Cisticola is a sedentary and year-round territorial species that inhabits open areas covered by tall grass, Common Bracken (Pteridium aquilinum), bushes, and rank vegetation along forest edges, clearings, and streams in the Afrotropical highlands (Ryan 2020). Birds spend most of their time in dense herbaceous vegetation, where they are difficult to observe. In Cameroon mountains Chubb’s Cisticola breeds in the dry season (from November to March). The birds are probably monogamous, with females leading the nest building and incubation, usually placing the nests 0.5–2.0 m above ground (Ryan 2020).
The song of Chubb’s Cisticola is described as a rapid, antiphonal duet or chorus initiated by a male and produced from an elevated point (Ryan 2020). Prior to this study, there was relatively little and uncertain knowledge on which part of the duet or chorus is sung by males and females, who takes part in the chorus, and the relative abundance of solos, duets, and choruses (Thorpe et al. 1972; Ryan 2020).
In this study we focused on three main questions related to joint singing behavior in Chubb’s Cisticola: (1) What is the acoustic structure of vocal elements produced by males and females in duets and choruses? (2) How often do birds produce solos, duets, and choruses? (3) Do the proportions of solos, duets, and choruses change across the year? The answers to these questions shed light on the potential functions of solos, duets, and choruses in Chubb’s Cisticola, and illuminate how studies focused only on a single stage of a species’ life-cycle (breeding season versus the whole year) may change our perception of that species’ behavior. To the best of our knowledge, our study is the first to provide a detailed description of the vocalizations of Chubb’s Cisticola, and one of only a few to analyze collective singing behavior over the course of a year.
Methods
Study site
The study was conducted in the Bamenda Highlands (part of the Cameroon Mountains Endemic Bird Area), near the village of Big Babanki in the Northwest Region of Cameroon. In the Bamenda Highlands two seasons occur: a shorter dry season from November to March and a longer, wet season from April to October (Tye 1986). We surveyed an unprotected area within 1 km of the field station (coordinates: N 6.0902° E 10.2948°). The altitude of the study site ranged from ca. 2000 to 2200 m a.s.l. The vegetation was composed of a mosaic of small fragments of montane rain forest, woodland dominated by Gnidia glauca, forest clearings, tree-covered corridors along streams, shrubs, extensive pastures, and abandoned lands covered by ferns (Pteridium aquilinum) and shrubs. Chubb’s Cisticola is observed mainly in abandoned lands covered by ferns and shrubs, pastures with shrubs, and along forest edges and clearings.
Fieldwork
We conduct our study from November 17, 2015, to November 28, 2016, to capture singing activity of Chubb’s Cisticola across whole year. In the first stage of the study, we attempted to recorded vocalizations of the species in different territories. We did this from November 17 to December 9, 2015—a period that corresponds to Chubb’s Cisticola breeding season, as well as that of most bird species within the study area (Tye 1992; Sedláček et al. 2007). We confirmed that Chubb’s Cisticola breeds in the dry season, as we found nests with eggs or chicks, and observed fledglings during the period of the study. However, we also observed territorial birds without symptoms of breeding, which suggests that nesting may extend over a longer period (Ryan 2020). Chubb’s Cisticolas were recorded mainly in the morning (06:00–11:00) and in the evening (from 16:00 to 18:00), from a distance up to 20 m away. We visited territories on two or three successive days and recorded spontaneously singing individuals using a directional microphone (Sennheiser ME67 with power module K6) connected to a digital recorder (Marantz PMD 620, 16-bit PCM.wav recording format, 48 kHz sample rate). In most territories (22 out of 25 visited) at least one individual had been marked with a color ring, thus we were sure that we recorded the same group. After each recording, we marked the position of the recorded birds using a GPS receiver. We used these high-quality manual recordings to characterize the song structure of Chubb’s Cisticola during the breeding season.
Previous work on Chubb’s Cisticola has not been able to unambiguously determine which phrases of a song are sung by each sex. Therefore, we captured birds using ornithological mist nets, marked them with color rings, and took blood samples to determine their sex. Blood was taken from a wing vein and applied to Whatman FTA Classic Cards. Birds were then released in good condition in the place of capture. In total, we individually marked from one to three individuals in each of 22 territories (46 individuals: 25 males and 21 females). The sex of captured birds was determined using molecular techniques (see Supplementary material 1 for more details). Color-marked birds were then recorded using a digital camera (Canon XA20) connected with a directional microphone (Sennheiser ME67 with K6 power module). We used playback to stimulate birds to sing and approach the camera. Chubb’s Cisticola sings very rarely and it is difficult to predict from which point within the territory the birds will start singing, thus we obtained only five good quality video recordings of duets and choruses (i.e., made from short distance, where bill movements of every individual are clearly visible) from five different territories in which at least one individual was individually marked by color rings. Out of them in three territories male and female were marked, in one territory two males and one female and in another one only a female was individually marked. Additionally, we recorded two males and three females which sung solo. These data were used to determine which notes of a song are sang by males and females.
Soundscape recording
We recorded soundscapes in six recording sites using six Song Meter SM3 autonomous sound recorders (Wildlife Acoustics) with two built-in internal omnidirectional microphones (SMM-A1; signal-to-noise ratio > 68 dB). Recorders were placed on trees, ca. 2–5 m above the ground, in the middle of six different territories of Chubb’s Cisticola. Previous studies showed that Song Meter SM3 can record songs of songbirds from distance up to 100–150 m (Yip et al. 2017). The linear distance between neighboring recorders ranged from 267 to 741 m; however, the mountainous terrain always ensured that there was no overlap between soundscapes recorded at neighboring sites. In each recording site, we recorded the soundscape from one hour before sunrise to one hour after sunset (“recording session”), once every seven days from December 7, 2015, to November 28, 2016. Thus, over the course of the year we collected 52 recording sessions in each recording site. We used the same settings in each recorder: 16-bit PCM.wav recording format, 48 kHz sample rate, stereo recording, no low or high pass filters. We used these data to examine changes in the proportions of solos, duets, and choruses across the year.
Acoustic analyses
We analyzed recordings using Avisoft SASLab Pro 5.1.17 (Avisoft Biacoustics) software, with the following settings: FFT = 512; Frame size = 75%; Window = Hamming; Temporal Overlap = 87.5%. These settings produced a sonogram of 163 Hz bandwidth with 94 Hz frequency and 1.33 ms temporal resolution.
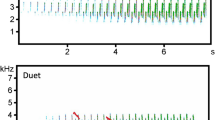
Song of Chubb’s Cisticola consists of syllables which are repeated many times in a synchronized manner. We defined syllables as: (1) solo when contained only notes produced by male or female; (2) duet, when contained notes produced by two individuals of the same or different sex; (3) chorus when contained notes produced by three or more individuals (Fig. 1). In this way, a song might contain from one (solo or duet or chorus) to three different types of syllables (solo, duet, and chorus).
Manual recordings
We first used video recordings of individually marked birds of known sex to determine which notes in the duets and choruses are sung by males and which are produced by females. Then we manually measured each song’s duration, counted the number of syllables in each song, and classified each syllable to one of three main types: solo, duet, or chorus. In the next step we classified syllable types into subtypes: (1) male solo (M) or female solo (F); (2) duet syllables were classified as female–male duet (FM), male–male duet (MM), or female-female duet (FF); and chorus syllables were classified according to the number and sex of participants [e.g., female–female–male chorus (FFM), male–male–female chorus (MMF), female–female–male–male chorus (FFMM), and so on]. We did not focus on the order in which the notes appeared in the syllable, thus abbreviation MMF means that elements produced by two males and one females are present in syllable, but it does not mean that the singing order is male–male–female. We also calculated the syllable rate within a song, measured as the number of syllables produced per second (number of syllables/song duration).
To characterize the general structure and variation of syllables produced by males and females we first visually classified each duet syllable to type. Then, we chose one high-quality example of each type of syllable (in total 35 syllables; see Appendix 2) and manually measured the duration (from the beginning of the first to the end of the last note) and minimal and maximal frequency of male and female syllables, counted notes in each syllable and measured duration and minimal and maximal frequency of each note separately in Avisoft SASLab Pro 5.1.17. We applied different settings to measure the temporal (FFT = 512; Frame size = 75%; Window = Hamming; Temporal Overlap = 87.5%) and spectral (FFT = 1024; Frame size = 75%; Window = Hamming; Temporal Overlap = 87.5) characteristics.
Soundscape recordings
We visually scanned spectrograms and selected good quality songs, i.e., songs in which we were able to see and recognize all notes within each syllable. We started scanning one hour before sunrise and stopped when we had found 100 syllables of Chubb’s Cisticola. On average, we found 100 good quality syllables in up to 4.34 h after sunrise (from 1 to 11 h after sunrise, SD 3.57); this time was shorter in the dry and at beginning of wet season (December–April; from 2.2 h in February to 2.96 h in April) and longer in the wet season (May–November from 4.3 h in May–July to 7.8 h in September). This way we ensured that the same number of syllables were analyzed at each recording site and during each recording session, and followed the natural singing activity of Chubb’s Cisticola. As with the manual recordings, we measured song duration, counted the number of syllables within a song, and classified each syllable to a type and subtype. Sound recordings are available at https://doi.org/10.7479/2j13-v254.
Statistical analyses
We used Mann–Whitney test to compare the differences in duration, minimal frequency, and frequency bandwidth of syllables and notes produced by males and females. We analyzed the 35 different types of syllables identified in our study. Because the manual recordings for each territory contained a different number of songs and syllables, we first calculated the average characteristics of each song (song duration, number of syllables within a song, syllable rate within a song) and the proportions of particular types and subtypes of syllables for each territory. We used these values to characterize song structure during the breeding season.
The year-round recordings were a typical example of repeated measurements: we recorded soundscapes in 6 recording sites, with 52 sessions in each site, and we analyzed the same number of syllables per recording session (100 syllables). To answer our question of whether the proportion of a particular type of syllable (solos, duets, choruses) changes across the year, we applied generalized estimating equations (GEE), which enable analysis of repeated measurement data and are marginal models that estimate population-averaged effects (Liang and Zeger 1986; Pekár and Brabec 2018). For each type of vocalization, we created a separate GEE. In each model, we specified recording site as a subject variable, recording session as the repeated measurement, and month as a fixed effect (nominal variable; as a reference category we used the month with the highest mean number of the type of vocalization under consideration). Data were fitted using a negative binomial distribution and log-link function. All p values are two-tailed. Statistical analyses were performed using IBM SPSS Statistics 26.
Results
Singing behavior
We observed Chubb’s Cisticola in groups of two to seven individuals. Birds sang from elevated places, such as shrubs, trees, or ferns, with no specific acoustic signal preceding singing. Birds appeared rapidly at the song post, sang one or more songs, and hid in the vegetation again. While singing together, birds were always in sight of one another and performed dance-like movements such as moving their tails and flapping their wings to produce loud wing-snaps, which suggests that duet and chorus coordination may be facilitated by visual signals (Supplementary material 2). We never observed any duet or chorus in which birds were further than 1 m apart while singing.
The syllables produced by females and males were sex-specific and possible to discrimination by manual spectrogram scanning. Analysis of 35 types of syllables recorded in 25 territories revealed that the syllables produced by females contained on average 2.9 whistling notes (from 2 to 4), while males produced syllables containing on average 3.2 decreasing in frequency notes (from 1 to 5). Syllables produced by females were significantly longer (female: average 0.50 s, from 0.353 to 0.707 s; male: average 0.43 s, from 0.114 to 0.509 s; Mann–Whitney test: Z = − 3.354, p < 0.001), produced in lower minimal frequency (female: average 2.0 kHz; from 1.55 to 3.57 kHz; male: average 2.7 kHz, from 1.89 to 4.26 kHz; Mann–Whitney test: Z = − 5.612, p < 0.001) but did not differ in frequency bandwidth (female: average 4.6 kHz; from 3.10 to 6.72 kHz; male: average 4.8 kHz, from 1.64 to 6.29 kHz; Mann–Whitney test: Z = − 1.386, p = 0.166) from male syllables (Fig. 1; Supplementary material 3). The duet comprised solo male and female syllables sung by both birds at the same time with high precision (Fig. 1). The song was composed of these same syllables repeated many times during singing (Fig. 1).
When we looked at the notes sung by males and females, we found that females produced significantly longer notes (female: average 0.13 s, from 0.021 to 0.29. sec; male: average 0.08 s, from 0.043 to 0.133 s; Mann–Whitney test: Z = − 3.047, p < 0.01), in lower minimal frequency (female: average 2.9 kHz; from 1.55 to 6.15 kHz; male: average 3.9 kHz, from 1.89 to 6.20 kHz; Mann–Whitney test: Z = − 6.312, p < 0.001) but did not differ in frequency bandwidth (female: average 2.3 kHz; from 0.17 to 4.91 kHz; male: average 2.0 kHz, from 0.51 to 3.53 kHz; Mann–Whitney test: Z = − 1.892, p = 0.059) from male notes. For more details, see Supplementary material 3.
Song structure during the breeding season
We recorded Chubb’s Cisticola in 25 different territories. In total, we analyzed 346 songs (on average 13.8 songs per territory; from 5 to 29 songs), containing 3625 syllables (on average 145.0 syllables per territory; from 28 to 257 syllables). The average song lasted 9.4 s (from 0.6 to 48.9 s) and contained on average 10.5 syllables (from 1 to 56). The average syllable rate was 1.1 syllables per second (from 0.6 to 1.7 syllables per second).
The most widespread type of song was duet produced by male and female, which was observed in all 25 visited territories. Choruses were recorded in 18 territories, while solos were observed in only one territory (one female sung one solo song comprised of three solo syllables). When we looked at the proportions of syllables found in solos, duets, and choruses within each territory, we found that, on average, 72.0% of syllables were duets, 27.9% were choruses, and 0.1% were solos (Fig. 2a).
The average proportion (per territory) during the breeding season of a duets, choruses, and solo syllables, and b various types of choruses: male–male–female (MMF), female–female–male (FFM), male–male–female–female (MMFF), and female–female–female–male (FFFM). Graph a is based on vocalizations recorded in 25 territories of Chubb’s Cisticola. Graph b is based on 18 territories in which at least one chorus syllable was recorded. Median, quartiles, outliers, and extreme values are given are given
We found four different types of choruses, each composed of three or four individuals: (1) two males and one female (MMF; 49.6% of syllables), (2) two females and one male (FFM; 44.9% of syllables), (3) two males and two females (MMFF; 5.2% of syllables), and (4) three females and one male (FFFM; 0.3% of syllables) (Figs. 2b, 3). From the 18 territories in which at least one chorus syllable was recorded, in 6 we observed only MMF chorus, in another 6 only FFM chorus, and in the remaining 6 we observed chorus syllables produced by three or four individuals. Supplementary material 4 contains the dataset of manual recordings collected during the breeding season.
Temporal changes in proportions of solos, duets, and choruses across the year
We analyzed 3213 songs, containing 31,200 syllables recorded in six recording sites during 52 recording sessions across the year. The average song contained 9.7 syllables (from 1 to 43). Of all syllables, 82.3% were duets, 15.8% choruses, and 1.9% solos. However, the relative frequency of solos, duets, and choruses changed over the course of the year (Fig. 4). See Supplementary material 5 for raw data.
The average number of solo, duet, and chorus syllables across the year. Graph is based on 52 recording sessions conducted in 6 recording sites. The average number of syllables per month per recording session and 95% confidence interval for the means are given. The breeding season in the study area is approximately from November to February
Solo vocalizations
Solo songs were recorded in each of the six recording sites, but with significant differences in the occurrence of solo syllables over time (Wald χ2 = 36.174; df = 11 p < 0.001) (Figs. 4, 5). The highest proportion of solo syllables was observed from August to October and in December, January, and April (Table 1, Fig. 4). Of the total 587 solo syllables recorded, 54.9% were sung by a female and 45.1% by a male. Female solo syllables were observed in 102 songs. In 73.5% of these, the song contained only female solo syllables (from 1 to 12). In the remaining cases, female solo syllables occurred within a song that also contained duet or chorus syllables, meaning that those solos occurred because the male did not follow the female’s syllable. Male solo syllables were observed in 135 songs, of which 32.6% comprised only male solo syllables (from 1 to 9). In the remaining cases, male solo syllables occurred within a song that also contained duet or chorus syllables, indicating that those solos occurred because the female did not follow the male’s syllable. The highest average proportion of solo male syllables was observed in April, while female solo syllables were most frequent in October (Fig. 5). The proportion of male and female solos varied between recording sites (Fig. 6). We found that number of solo syllables was independent of the time of day (Kruskal–Wallis test; df = 12, H = 15.628, p = 0.209).
The average number of solo female and solo male syllables across the year. Graph is based on 52 recording sessions conducted in 6 recording sites. The average number of syllables per month per recording session and 95% confidence interval for the means are given. The breeding season in the study area is approximately from November to February
Duets and choruses
Of a total of 25,695 duet syllables, 99.9% were between a male and a female. However, we also found 19 duet syllables between males and seven duet syllables between females. The highest proportion of duet syllables was observed from September to December (Fig. 4), but we found no significant differences in the proportion of duets among months (Wald χ2 = 2.418; df = 11; p = 0.996) (Table 1; Fig. 4).
Of a total of 4918 chorus syllables, the most common types were choruses of two males and one female (49.7%) and two females and one male (40.4%). However, we also found choruses of more than three individuals: two males and two females (9.4%), three males and one female (0.5%), three males and two females (2 syllables), and three females and one male (1 syllable). Chorus syllables were observed in 720 songs. In 11.8% of these, the song contained only chorus syllables (from 1 to 19). In the remainder of the cases, chorus syllables occurred within a song that also contained duet syllables, meaning that a third—and in some cases, a fourth—individual joined some syllables within a song. We found significant differences in the relative frequency of chorus syllables among months (Wald χ2 = 87.275; df = 11,300, p < 0.001), with the highest proportions observed from January to June (Figs. 4, 7, Table 1).
Discussion
In Chubb’s Cisticola, we documented interesting singing behavior in several respects. The first is that individually variable whistling notes were produced by females, while males produced trilling notes (Fig. 1). In most bird species, males produce vocalizations that are more complex than those of females and responsible for the repertoire of song (Catchpole and Slater 2008). Indeed, in the majority of duetting species, the most-common patterns are whistling notes from males and trilling notes from females [e.g., Yellow-breasted Boubou (Laniarius atroflavus); Wheeldon et al. 2020] or notes that are similar in structure from both sexes (Voigt et al. 2006; Bradley and Mennill 2009). Thus, our finding suggests that in Chubb’s Cisticola females tend to have vocalizations that are responsible for duet repertoire, while males produce simpler, less variable, trilling notes (Supplementary material 3). Two evolutionary paths may explain such a pattern: the loss of song complexity by males, as was observed in females in some species (Price et al. 2009), or strong selective pressure for song complexity in females (e.g., because the vocal role of a female in territory defense is more important than that of a male; Illes and Yunes-Jimenez 2009). In both cases, our finding provides new insights into the evolution of vocal complexity in animals. However to understand why such a phenomenon may have had the opportunity to evolve in Chubb’s Cisticola, we need more information about the biology and ecology of the species.
The genus Cisticola comprises 51 species (Winkler et al. 2020). In the majority of these, males sing species-specific songs containing trills, whistles, rasping, and atonal phrases. Some produce songs organized in a very stereotypical way (Benedict and Bowie 2009, 2012). Of these 51 species, six have also been reported to perform duets, and in three other the females occasionally produce rasping sounds during male singing, which can be classified as a simple form of duet (Winkler et al. 2020). This in itself is not unusual, since many groups of closely related species differ in their song structure and social behavior [e.g., barbets (Soma and Brumm 2020) or neotropical wrens (Keenan et al. 2020)]. Cisticola males and females are visually indistinguishable in the field and duets are fast and complex; for this reason, it has historically been difficult to determine which phrases are produced by a particular sex. Indeed, in Hunter’s Cisticola (Cisticola hunteri)—which has a song structure that is very similar to that of Chubb’s Cisticola—two different studies reported contrasting results with respect to the roles of males and females in duets (Todt 1970; Thorpe et al. 1972). Both studies did not specify how the sex of singing birds was determined and how particular notes were assigned to an individual. In our study, we recorded birds using a camera and sexed them using molecular methods, which enabled us to unambiguously show that males produce trills while females produce whistles. We thus suggest that it may be time to revise our knowledge about song structure in Chubb’s Cisticola and probably also in two other species of duetting cisticola: Hunter’s Cisticola and the Black-lored Cisticola (Cisticola nigriloris) (Ryan 2020).
Our song analyses point to the existence of interesting social structure in Chubb’s Cisticola. In addition to duets, we also observed choruses composed of three to five individuals, as first mentioned by Thorpe et al. (1972). Both two males and one female as well as two females and one male choruses were observed, in similar proportions (Fig. 2). In forming a chorus, the additional individual(s) tried to precisely match his or her own phrases to those of the same-sex individual (Fig. 3), which is one of the most complex types of singing performance described in animals. In most cases, the third individual sang along with a duet for only a few syllables within a longer song: the vast majority of songs that contained chorus syllables also had duet syllables, with only 11% of such songs composed solely of chorus syllables. Similar complex chorusing behavior, in which individuals join in and drop out of the chorus, has also been found in cooperatively breeding Plain-tailed Wrens (Thryothorus euophrys) (Mann et al. 2006) and White-browed Sparrow Weavers (Plocepasser mahali) (Voigt et al. 2006). In Chubb’s Cisticola, the proportion of chorus syllables increased over time until March and then started to decrease. Assuming that most pairs breed in November–December, we hypothesize that offspring take part in the chorus until March and then most of them leave the natal territory, resulting in a decrease in the frequency of choruses. A group structure characterized by the delayed dispersal of offspring is the most common behavior in avian societies (Hatchwell 2009). However, the fact that we observed choruses across the entire year (Fig. 4) suggests that some individuals may stay longer as helpers in the natal territory. A similar pattern was observed in the Toucan Barbet (Semnornis ramphastinus), in which groups were composed of a breeding pair and their offspring, and the size of the group was significantly smaller during the breeding season than during the non-breeding season (Restrepo and Mondragon 1998). Looking at year-round chorusing activity in Chubb’s Cisticola, it is important to note that the proportion of choruses depended heavily on the territory in which the soundscape was recorded (Fig. 7). Thus, a high proportion of choruses may also be an indicator of breeding success or territory quality (Brunton et al. 2016). However, further studies examining how singing in duet and chorus is coordinated and whether coordination level is a signal of group size and quality are still needed.
One of the most important functions of bird song is mate attraction (Catchpole and Slater 2008). In duetting species, intensive solo singing could be an advertising signal that communicates the mating status of the signaler (Topp and Mennill 2008). In Chubb’s Cisticola, we observed solo singing extremely rarely—only in 2% of year-round syllables. Such a low solo rate may indicate that pair bonding is a continuous process, in which nomads or helpers take over the position of one of the pair members after its death (Riehl 2013). Alternatively, a high number of nomads or helpers waiting for a vacant territory within a population may support such low solo singing activity. Looking at male and female solo activity, we found a striking difference between the sexes: 33% of songs with male solo syllables were composed only of such syllables, while the corresponding percentage for females rose to 74%. This means in the majority of songs with female solos, a male does not join in, which could suggest a leading role for females in acoustic territory defense (Illes 2015). Throughout the year, we also observed contrasting patterns of male and female solo activity. Females produced more solo syllables than males before and during the breeding season (September, October, December), while males produced more solo syllables than females after the breeding season (February and April) (Fig. 5). This could be an indication of sexual conflict between males and females and seasonal sex-specific variation in motivation to follow a mate’s song. However, it is important to note that the year-round proportion of male and female solo syllables varied strongly among territories (Fig. 6). Thus, the general pattern of solo singing activity seems to be strongly modified by random events taking place in particular territories.
Cooperative defense of resources is thought to be the main function of coordinated acoustic displays in birds (Tobias et al. 2016). Our study showed that in Chubb’s Cisticola territories were defended acoustically year-round, in most cases by two or more individuals (only 3.7% of songs contained only solo syllables). At the same time, year-round coordinated duets and choruses may also serve to maintain long-term pair bonds or signal the quality of a pair or group (Hall 2009). The precision of a coordinated duet or chorus may be a reliable means of signaling information about a group’s size, experience, or quality to other groups (Hall and Magrath 2007). Other putative functions of duets, such as maintaining contact and mate or paternity guarding, are unlikely in Chubb’s Cisticola. Both of these hypotheses assume that duetting individuals respond to each other in order to confirm their presence within the territory (Hall 2004). Although the intensity of response depends on the progression of the breeding stage (Topp and Mennill 2008), in these scenarios, communicating individuals are generally far away from each other, at least at the initial part of the duet (and thus have the need to confirm their presence). In Chubb’s Cisticola, individuals start singing a duet or chorus only when they are very close together. Such behavior suggests that maintaining contact is not the function of duets or choruses (Hall 2009) in Chubb’s Cisticola. However, to better understand the function of solos, duets and choruses in Chubb’s Cisticola, an experimental study involving a playback which examines the response of birds to various kinds of vocalizations in different contexts is needed.
Our study reveals the importance of looking at singing behavior on a broad temporal scale, especially in sedentary species. Here, a study focused only on the breeding season would have underestimated solo singing activity and completely missed the production of solo syllables by males. Moreover, by analyzing changes in the relative frequency of solos, duets, and choruses across the year we were able to see patterns which would have remained undetected in a survey conducted over a short period of the breeding season. Duets and choruses observed year-round suggest that coordinated singing is used primarily for resource defense. The other singing behaviors observed—cooperative singing only between individuals that are physically close and the relative rarity of solo songs—make other functions of duets and choruses, such as maintaining contact or paternity guarding, unlikely in Chubb’s Cisticola. However, to better understand the function of solos, duets and choruses in Chubb’s Cisticola more detailed studies are needed.
Data availability
The data underlying in this article are available as supplementary materials.
References
Baker MC (2004) The chorus song of cooperatively breeding laughing kookaburras (Coraciiformes, Halcyonidae: Dacelo novaeguineae): characterization and comparison among groups. Ethology 110:21–35
Baker MC (2009) Information content in chorus songs of the group-living Australian magpie (Cracticus tibicen dorsalis) in Western Australia. Ethology 115:227–238
Benedict L, Bowie RCK (2009) Macrogeographical variation in the song of a widely distributed African warbler. Biol Lett 5:484–487
Benedict L, Bowie RCK (2012) Rattling cisticola song features and variability across sub-Saharan Africa. J Zool 287:96–103
Benedict L, McEntee JP (2009) Context, structural variability and distinctiveness of california towhee (Pipilo crissalis) vocal duets. Ethology 15:77–86
Bradley DW, Mennill DJ (2009) Solos, duets and choruses: vocal behaviour of the Rufous-naped Wren (Campylorhynchus rufinucha), a cooperatively breeding neotropical songbird. J Ornithol 150:743–753
Brunton DH, Roper MM, Harmer AMT (2016) Female song rate and structure predict reproductive success in a socially monogamous bird. Front Ecol Evol 4:13
Catchpole CK, Slater PJB (2008) Bird song. Biological themes and variation, 2nd edn. Cambridge University Press, Cambridge
Dahlin C, Benedict L (2014) Angry birds need not apply: a perspective on the flexible form and multifunctionality of avian vocal duets. Ethology 120:1–10
Diniz P, da Silva Júnior EF, Webster MS, Macedo RH (2018) Duetting behavior in a Neotropical ovenbird: sexual and seasonal variation and adaptive signaling functions. J Avian Biol 49:jav-01637
DuVal EH (2007) Cooperative display and lekking behavior of the Lance-tailed Manakin (Chiroxiphia lanceolata). Auk 124:1168–1185
Grafe TU, Bitz JH (2004) An acoustic postconflict display in the duetting tropical boubou (Laniarius aethiopicus): a signal of victory? BMC Ecol 4:1
Hale AM (2006) The structure, context and functions of group singing in black-breasted wood-quail. Behaviour 143:511–533
Hall ML (2004) A review of hypotheses for the functions of avian duetting. Behav Ecol Sociobiol 55:415–430
Hall ML (2009) A review of vocal duetting in birds. Adv Stud Behav 40:67–121
Hall ML, Magrath RD (2007) Temporal coordination signals coalition quality. Curr Biol 17:R406–R407
Hatchwell BJ (2009) The evolution of cooperative breeding in birds: kinship, dispersal and life history. Phil Trans R Soc B 364:3217–3227
Illes AE (2015) Context of female bias in song repertoire size, singing effort, and singing independence in a cooperatively breeding songbird. Behav Ecol Sociobiol 69:139–150
Illes AE, Yunes-Jimenez L (2009) A female songbird out-sings male conspecifics during simulated territorial intrusions. Proc R Soc B 276:981–986
Keenan EL, Odom KJ, Araya-Salas M, Horton KG, Strimas-Mackey M, Meatte MA, Mann NI, Slater PJB, Price JJ, Templeton CN (2020) Breeding season length predicts duet coordination and consistency in Neotropical wrens (Troglodytidae). Proc R Soc B 287:20202482
Klenova AV, Volodin IA, Volodina EV (2008) Duet structure provides information about pair identity in the red-crowned crane (Grus japonensis). J Ethol 26:317–325
Koloff J, Mennill DJ (2012) Vocal behaviour of Barred antshrikes, a neotropical duetting suboscine bird. J Ornithol 154:51–61
Leighton GM (2017) Cooperative breeding influences the number and type of vocalizations in avian lineages. Proc R Soc B 284:20171508
Liang K-T, Zeger SL (1986) Longitudinal data analysis using generalized linear models. Biometrika 73:13–22
Logue DM, Hall ML (2014) Migration and the evolution of duetting in songbirds. Proc R Soc B 281:20140103
Mann NI, Dingess KA, Slater PJB (2006) Antiphonal four-part synchronized chorusing in a Neotropical wren. Biol Lett 2:1–4
Mennill DJ, Vehrencamp SL (2005) Sex differences in singing and duetting behavior of neotropical Rufous-and-white wrens (Thryothorus rufalbus). Auk 122:175–186
Mennill DJ, Vehrencamp SL (2008) Context-dependent functions of avian duets revealed by microphone-array recordings and multispeaker playback. Curr Biol 18:1314–1319
Moore IT, Bonier F, Wingfield JC (2005) Reproductive asynchrony and population divergence between two tropical bird populations. Behav Ecol 16:755–762
Odom K, Hall M, Riebel K, Omland K, Langmore NE (2014) Female song is widespread and ancestral in songbirds. Nat Commun 5:3379
Odom KJ, Logue DM, Studds CE, Monroe MK, Campbell SK, Omland KE (2017) Duetting behavior varies with sex, season, and singing role in a tropical oriole (Icterus icterus). Behav Ecol 28:1256–1265
Pekár S, Brabec M (2018) Generalized estimating equations: a pragmatic and flexible approach to the marginal GLM modelling of correlated data in the behavioural sciences. Ethology 124:86–93
Price JJ, Lanyon SM, Omland KE (2009) Losses of female song with changes from tropical to temperate breeding in the New World blackbirds. Proc R Soc B 276:1971–1980
Restrepo C, Mondragon ML (1998) Cooperative breeding in the frugivorous toucan barbet (Semnornis ramphastinus). Auk 115:4–15
Riehl C (2013) Evolutionary routes to non-kin cooperative breeding in birds. Proc Biol Sci 280:20132245
Ryan P (2020) Chubb’s Cisticola (Cisticola chubbi), version 1.0. In: del Hoyo J, Elliott A, Sargatal J, Christie DA, de Juana E (eds) Birds of the world. Cornell Lab of Ornithology, Ithaca
Seddon N (2002) The structure, context and possible functions of solos, duets and choruses in the subdesert mesite (Monias benschi). Behaviour 139:645–676
Seddon N, Tobias JA (2003) Communal singing in the cooperatively breeding subdesert mesite (Monias benschi): evidence of numerical assessment? J Avian Biol 34:72–80
Sedláček O, Reif J, Hořák D, Riegert J, Pešata M, Klvaňa P (2007) The birds of a montane forest mosaic in Big Babanki area, Bamenda Highlands, Cameroon. Malimbus 29:89–100
Soma M, Brumm H (2020) Group living facilitates the evolution of duets in barbets. Biol Lett 16:20200399
Templeton CN, Rıos-Chelen AA, Quiros-Guerrero E, Mann NI, Slater PJB (2015) Female happy wrens select songs to cooperate with their mates rather than confront intruders. Biol Lett 9:20120863
Thorpe WH, Hall-Craggs J, Hooker T, Hutchison R (1972) Duetting and antiphonal song in birds: its extent and significance. Behav Suppl 18:III–197
Tobias JA, Sheard C, Seddon N, Meade A, Cotton AJ, Nakagawa S (2016) Territoriality, social bonds, and the evolution of communal signaling in birds. Front Ecol Evol 4:74
Todt D (1970) Die antiphonen Paargesänge des ostafrikanischen Grassängers Cisticola hunteri prinioides Neumann. J Ornithol 111:332–356
Topp SM, Mennill DJ (2008) Seasonal variation in the duetting behaviour of rufous- and-white wrens (Thryothorus rufalbus). Behav Ecol Sociobiol 62:1107–1117
Tye H (1986) The climate of the highlands of Western Cameroon. In: Stuart SN (ed) Conservation of Cameroon montane forests. Report of the ICBP Cameroon montane forest survey. International Council for Bird Preservation, Cambridge, pp 18–19
Tye H (1992) Reversal of breeding season by lowland birds at higher altitudes in western Cameroon. Ibis 134:154–163
Voigt C, Leitner S, Gahr M (2006) Repertoire and structure of duet and solo songs in cooperatively breeding white-browed sparrow weavers. Behaviour 143:159–182
Voigt C, Leitner S, Gahr M, Maat AT (2021) Seasonal and diurnal variation of vocal behaviour in duetting White-browed Sparrow Weavers. J Ornithol 162:1163–1172
Vokurková J, Motombi FN, Ferenc M, Horák D, Sedlácˇek O (2018) Seasonality of vocal activity of a bird community in an Afrotropical lowland rain forest. J Trop Ecol 34:53–64
Wheeldon A, Szymański P, Budka M, Osiejuk TS (2020) Structure and functions of Yellow-breasted Boubou (Laniarius atroflavus) solos and duets. PeerJ 8:e10214
Winkler DW, Billerman SM, Lovette IJ (2020) Cisticolas and allies (Cisticolidae), version 1.0. In: Billerman SM, Keeney BK, Rodewald PG, Schulenberg TS (eds) Birds of the world. Cornell Lab of Ornithology, Ithaca
Wright TF, Dahlin CR (2007) Pair duets in the yellow-naped amazon (Amazona auropalliata): phonology and syntax. Behaviour 144:207–228
Yip DA, Leston L, Bayne EM, Sólymos P, Grover A (2017) Experimentally derived detection distances from audio recordings and human observers enable integrated analysis of point count data. Avian Conserv Ecol 12:11
Acknowledgements
We thank the Kedjom-Keku community, and particularly Ernest Vunan, for their help organizing the fieldwork and their kind reception in Big Babanki. We gratefully acknowledge Marcin Antczak, Ania Skierczyńska, Michał Skierczyński, and Piotr Chmielewski for their help in the field, Lindsay Higgins and Agata Staniewicz for the language correction of the manuscript.
Funding
This work was supported by the Polish Ministry of Science and Higher Education (Grant Iuventus Plus, number IP2014 005073).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethics approval
The experimental procedure was approved by The Poznań Local Ethics Committee for Animal Experimentation (decision number 16/2015). We got permission from the Kedjom-Keku community to conduct the study, including access to the study site and permission to camp in the mountains.
Additional information
Communicated by F. Bairlein.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Budka, M., Piasecka, M., Białas, J.T. et al. Frequent duets, rare choruses, and extremely rare solos: year-round singing behavior in Chubb’s Cisticola. J Ornithol 164, 547–559 (2023). https://doi.org/10.1007/s10336-023-02052-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10336-023-02052-0