Abstract
In many bird species, physical aggression between males become more frequent during the female’s fertile period, as female encounters with extra-pair males are more frequent and can entail paternity losses. Male aggressiveness during this stage has been proposed as crucial for ensuring male reproductive success. Thus, plumage ornaments could represent honest signals of individual quality that could reflect the aggressiveness of paired territorial males. Furthermore, male aggressiveness could be related to mate quality or defensive capacity. We quantified extra-pair paternity in the broods and investigated the association of male and female traits with the aggressive behaviour of territorial paired males in a Spanish population of Pied Flycatchers (Ficedula hypoleuca), where territorial intrusions were simulated during the female fertile period by placing a taxidermic male mount close to the nest. We predicted that (1) more aggressive males should better protect their mates from intruding males and thereby reduce their paternity losses, (2) males with larger white patches and higher UV reflectance of wing patches should respond more strongly to intrusions, and (3) that males should be more aggressive when mated with higher quality females. We found evidence that males that responded less intensely to a territorial intrusion suffered a higher paternity loss, which offers strong support to the basic tenet of the theory of territoriality as paternity defence. Moreover, both the level of male aggressiveness and control of the territory increased with male UV reflectance of wing patches. Finally, we found, contrary to our prediction, that males were less aggressive when mated with more ornamented females.
Zusammenfassung
Männliche Aggressivität während der weiblichen fertilen Phase in Relation zu Fremdvaterschaften, Gefiederornamenten und Merkmalen der Weibchen
Bei vielen Vogelarten nimmt die Häufigkeit physischer Aggression zwischen Männchen während der fertilen Phase der Weibchen zu, wenn Zusammentreffen der Weibchen mit fremden Männchen häufiger sind und zu Vaterschaftsverlusten führen können. Die Aggressivität der Männchen in diesem Stadium gilt als entscheidend für die Sicherung ihres Fortpflanzungserfolgs. Somit könnten Gefiederornamente ehrliche Signale individueller Qualität darstellen, welche die Aggressivität verpaarter Reviermännchen widerspiegeln. Außerdem könnte die männliche Aggressivität in Verbindung mit der Qualität der Partnerin oder den Verteidigungsfähigkeiten stehen. An einer spanischen Population von Trauerschnäppern Ficedula hypoleuca bestimmten wir den Anteil von Fremdvaterschaften an den Bruten und untersuchten den Zusammenhang männlicher und weiblicher Merkmale mit dem Aggressionsverhalten verpaarter Reviermännchen, indem wir während der fertilen Phase der Weibchen durch Platzieren eines präparierten Trauerschnäppermännchens in Nestnähe eine Übertretung der Reviergrenze simulierten. Wir sagten vorher, dass: 1) aggressivere Männchen ihre Partnerinnen besser vor Eindringlingen beschützen und so ihre Vaterschaftsverluste reduzieren; 2) Männchen mit größeren weißen Flecken und stärkerer UV-Reflexion der Flügelfelder stärker auf Eindringlinge reagieren und dass 3) mit Weibchen höherer Qualität verpaarte Männchen aggressiver sind. Wir fanden Belege dafür, dass Männchen, welche schwächer auf eine Überschreitung der Reviergrenze reagierten, höhere Vaterschaftsverluste zu tragen hatten, was eine klare Bestätigung der Grundsatzthese ist, dass Territorialität der Vaterschaftsverteidigung dient. Außerdem nahmen sowohl die männliche Aggressivität als auch die Kontrolle über das Revier in Abhängigkeit von der UV-Reflexion der Flügelfelder bei den Männchen zu. Zu guter Letzt stellte sich entgegen unserer Erwartungen heraus, dass Männchen weniger aggressiv waren, wenn sie mit stärker ornamentierten Weibchen verpaart waren.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Male territorial aggression is considered an adaptive response to competitors as it improves access to food resources, breeding territory and/or mates (Brown 1964, 1969). Male aggressiveness during their mates’ fertile period has been proposed as crucial for ensuring male reproductive success. It is predominantly during this period that physical aggressions between males become more frequent in many species (Birkhead and Møller 1992). This is because engaging in extra-pair copulations (EPCs) has an evident benefit for males, as extra‐pair paternity (EPP) provides a way to increase reproductive success without the costs of care (Nakagawa et al. 2015). Thus, female encounters with extra-pair males during the fertile period represent the most frequently observed extra-pair events in birds (Westneat and Stewart 2003). Social males have therefore evolved behaviours through which they minimize the risks of cuckoldry and protect their paternity, like mate guarding or territory defence (Griffith et al. 2002; Møller and Birkhead 1991). Through these behaviours, the social male tries to interfere with EPC attempts by intruding males (Birkhead and Møller 1992; Westneat 1994). Therefore, mate guarding and male aggressive behaviours are often most intense during the female’s fertile period (Birkhead and Møller 1992). While aggressive behaviours during this phase seem to involve costs like increased energy expenditure, reduced feeding opportunities and high risk of predation and physical injury (Birkhead and Møller 1992; Komdeur 2001; Low 2006; Wingfield et al. 1990, 2001), they also provide crucial benefits in terms of paternity protection (Møller and Birkhead 1991). Consequently, the most aggressive and territorial males should receive the greatest benefits. Thus, males that experience a greater loss of paternity might not be effective in protecting their partner from the approach of intruding males (Low 2005).
Males of many bird species present different traits that determine success in intrasexual competition, such as large body size, weaponry (bills, talons), and several showy ornaments and sexual signals used in aggressive displays (Andersson 1994; Darwin 1871). The conspicuousness of some male traits represents honest signals of individual quality and competitive abilities that can help to solve a conflict without costly fighting (Plaza et al. 2018; Rohwer and Ewald 1981). Thus, signalling individuals could avoid costly aggressive interactions with high-quality opponents and reduce the risk of being injured during agonistic encounters that may reduce survival and limit parental care (Wingfield et al. 1990, 2001). Although carotenoid-based plumage and tegumental ornamentation are largely studied as honest signals used in intra- and inter-sexual interactions (reviewed in Hill 1999; Griffith et al. 2006), melanin-based plumage ornaments are more involved in male–male competition as signals of male dominance, territoriality and/or fighting abilities (Jawor and Breitwisch 2003; reviewed in Senar 1999). In addition, recent studies of variation in unpigmented feather patches suggest a signalling function in social interactions for white plumage and its UV colouration that could be used during the breeding and non-breeding seasons (Cantarero et al. 2017; Morales et al. 2014).
In several species of birds, females develop secondary sexual characteristics comparable to those of males, including plumage ornaments (Amundsen 2000; Andersson 1994). Therefore, it is plausible that a female can honestly report its quality to its mate through plumage traits exactly in the same way as males. Thus, males may be more prone to defend their territory and paternity when paired with high-quality females as evidenced by dominance signals. On the other hand, dominant or strong females may be more capable of defending themselves from unwanted suitors (Moreno et al. 2014) and require less protection from their mates in a context of conflict between the reproductive interests of females and extra-pair males (Arnqvist and Kirkpatrick 2005; Westneat and Stewart 2003). In both scenarios, both behavioural and morphological traits of the female could influence the level of male aggressiveness during the fertile phase.
Here, we studied male territorial aggression during the fertile phase of mates in a Spanish population of the Pied flycatcher (Ficedula hypoleuca), a small hole-nesting migratory passerine which shows a moderate level of EPP (Plaza et al. 2019a, b), making this species a good model to study the evolution of male mate guarding. Male and female Pied Flycatchers differ in the expression of melanin-based dorsal plumage (Lundberg and Alatalo 1992), with males showing a highly contrasting black and white plumage and a white forehead patch in contrast with the duller brown and white colour of the females (Lehtonen et al. 2009a). Both sexes have conspicuous white wing patches characterized by different levels of UV reflectance (Lundberg and Alatalo 1992). Males with larger white forehead patches are more likely to win aggressive encounters in disputes for nest boxes (Järvistö et al. 2013; Silverin 1998). Therefore, more ornamented males should have more capacity to ensure paternity, whereas less dominant males should consequently suffer increased levels of EPP (Low 2005). Indeed, a previous study has found that males that were less aggressive during playback territorial intrusion tests performed during the female fertile period suffered a higher paternity loss (Moreno et al. 2010), whereas less ornamented males were less aggressive towards intruders (Moreno et al. 2010, 2015). However, no study has investigated the association of UV reflectance of white wing patches with male territoriality. In a Finnish Pied Flycatcher population, the contrast between UV colouration of white wing patches and its background has been correlated with mate choice (Sirkiä and Laaksonen 2009), and the proportional UV reflectance of dark feathers is higher in early arriving males than in those arriving later (Siitari and Huhta 2002). Therefore, the UV component of male colouration may signal individual quality and be associated with mate guarding and nest defence in this species. In addition, as with incubation feeding, even the level of aggressiveness may be influenced by the mate’s phenotypic characteristics (Kötél et al. 2016). In this species, females with larger wing patches and higher UV reflectance breed earlier and have higher testosterone levels during incubation (Cantarero et al. 2017, 2015; Morales et al. 2007; Moreno et al. 2014). It has also been demonstrated that the white forehead patch is involved in female–female aggressive interactions and as badge of oxidative status (Morales et al. 2014; Moreno et al. 2013). Therefore, female ornaments could also be a signal of individual condition, and males may be more aggressive towards intruders when paired with high-quality females, thereby reducing the probability of EPP.
We simulated territorial intrusions during the female fertile period by placing a stuffed Pied Flycatcher male close to the nest to explore the association between male and female ornaments and the aggressive behaviour of the males. We assumed that the male that protected the female from intruding males during her fertile period through aggression at her nest box is the same individual that subsequently bred with that female in that same nest box. We also analysed the paternity of the territorial pairs’ brood. We developed several predictions that represent possible scenarios of how male aggressiveness during the female fertile phase is related to extra-pair paternity, plumage ornaments and female traits. In particular, we predicted that in a context of EPP where the ability of the male to defend its territory could play an important role as a paternity guard, (1) more aggressive males should better protect their mates from intruding males and thereby suffer a lower incidence of EPP. In addition, if plumage signals honestly signal male competitive ability (Andersson 1994; Zahavi 1975), (2) more ornamented males (larger white patches and with higher UV reflectance) should respond more strongly to another male’s intrusion. Finally, if female plumage ornaments are also signals of individual quality, (3) males should be more aggressive when mated with higher quality females.
Material and methods
General field methods
The study was conducted during the breeding season of 2015 in a montane forest of Pyrenean oak Quercus pyrenaica, situated at 1200 m above sea level, near the village of Valsaín, Central Spain (40° 54ʹ N, 4° 01ʹ W). In this study area, where passerine birds breeding in nest boxes have been studied since 1991 (see Sanz et al. (2003) for general description), 300 nest boxes (see Lambrechts et al. (2010) for structural characteristics and placement of nest boxes) are routinely monitored during the Pied Flycatcher breeding season, lasting from the arrival of the first male in the area to the fledging of the last chicks (approximately from the middle of April to early July). Nest boxes were checked every 4 days to monitor the nest construction status for each breeding pair and, afterwards, the laying date was recorded by checking the nests occupied by Pied Flycatchers every 3 days. In addition to clutch size, also hatching dates and brood sizes at hatching and fledging were determined.
Simulated territorial intrusion tests and video recording
The aggressiveness tests were performed by simulating territorial intrusions by other males on the days after the nest was fully constructed, a period which overlaps in time with the fertile period (Birkhead et al. 1997). For this, we used two stuffed Pied Flycatcher males, one of which was randomly assigned to each nest prior to the test and placed inside a cage prebuilt with a metal structure and covered with a net to prevent damage to the decoy by the focal male (Fig. 1). The cage was placed hanging from a branch attached to a metal hook at an approximate distance of 0.5 m from the nest. Stuffed males used in this experiment were individuals belonging to the same population that were found dead in previous years and that were preserved at − 20 °C prior to preparation. The aggressive responses towards the stuffed males (see below) were similar regardless of the decoy used (all p values > 0.133). Each experimental system (n = 64), which includes the nest box, the decoy and their surrounding area, was filmed for 30 min (mean ± SE = 33.33 ± 3.14 min) with digital video cameras (Sony Dcr-sr190, with an extra battery Sony np-fh100) placed 50 m away from the nest box tree and recording an area of approximately 3 m around the nest box. The recording was activated immediately after the experimental cage was placed and all the videos were filmed between 8.00 and 11.00 am.
Capture and sampling
All adults (n = 125) were captured in their nest boxes with traps while feeding 7-day-old nestlings. Traps were active for no more than 1 h to minimize disturbance. They are metal flaps that close the nest entrance when activated by a visiting bird. Once the individual had been captured, it was identified by its ring, or ringed if necessary, and the wings were positioned in their extended positions to take digital photographs of the white wing patches. At the same time, a digital photograph of the white forehead patch was also taken. In addition, the percentage of black feathers on the head and mantle of males was recorded. This trait was scored as “blackness” on a 0–100 scale with 10% interval scores (Moreno et al. 2010, 2015). The tarsus length, the wing length and the body mass were also recorded. For all these procedures, the same methodologies used in previous studies carried out on the same Iberian population of Pied Flycatcher were applied (Cantarero et al. 2015; Moreno et al. 2010, 2015; Plaza et al. 2019a, b).
The exact age was known for individuals that were ringed as nestlings in the study area. Males were classified according to Jenni and Winkler (1994) and Svensson (1984) as first-year birds or older from the colour of primaries and primary coverts (brown in 1-year birds). Immigrant birds at first capture were assigned a minimum age of 2 years (Potti and Montalvo 1991). Once they have bred in our nest boxes, surviving males and females return each spring to attempt breeding.
Moreover, following Lehtonen et al. (2009b), the central tertial feather from the centre of the white patch of each wing was collected from each individual to subsequently measure the UV reflectance (see below). We chose to measure the UV reflectance of the white part, as the achromatic feathers typically show a relatively higher UV reflectance than the pigmented ones (Eaton and Lanyon 2003). Finally, a small blood sample from the brachial vein was collected for each adult individual and stored on Flinders Technology Associates reagent loaded cards (Whatman Bioscience, Florham Park, NJ, USA) for subsequent paternity analyses.
All chicks were ringed when they were 13 days old (hatching day = day 1). Tarsus length, wing length and body mass were measured using the same procedure described for adults. In addition, a small blood sample from the brachial vein was similarly collected for paternity analyses. All carcasses and abandoned eggs with an embryo inside found in nest boxes during routine monitoring were collected and frozen on the same day for subsequent tissue extraction and paternity testing.
UV reflectance
The protocol used by Lehtonen et al. (2009a) was taken as a reference to obtain the UV reflectance data in males (n = 55) and females (n = 58). This protocol provides for the measurement of reflectance in the UV portion of the spectrum by calculating UV chroma as the reflectance in the UV range divided by total reflectance in the avian visual range (R320–400 nm/R320–700 nm). A spectrophotometer under standardized laboratory conditions was used to obtain the UV measurements on the white area of the central tertial feather of each wing, using a white standard as control. To avoid background interference during the process, the feathers were placed on a low-reflectance black cloth. The measurement was carried out at three different points of the white part of the feathers in all males and females. UV reflectance values of the left and right wing were significantly correlated in both males (r = 0.36, p value = 0.006) and females (r = 0.41, p value < 0.001); therefore, for each individual, the average between these two values was considered.
Photo analysis
Digital photographs were analysed with Adobe Photoshop CS5 v.11.0 to estimate the sizes of white patches (zoom of 400% and paintbrush of 17 pixels, with 100% hardness and 25% spacing; Sirkiä et al., 2015). The values of white patches areas were given in square centimetres, and the distance of 1 mm on the ruler was used to calibrate the measurement.
Genetic analyses
We collected samples from 64 broods at 13 days of age and their two attending mates (125 adults, 304 nestlings). EPP analyses were performed following the protocol used in Plaza et al. (2019a) and, for each nest, two variables were calculated from the paternity analysis: presence/absence of EPP (indicated by 1 and 0, respectively) and the proportion of EPP (proportion of the brood fathered by males other than the attending male).
Behavioural data analysis
All recordings (n = 64) were viewed with VLC Media Player software always by the same observer (MB). As a measure of male territoriality, “latency” was defined as the time in seconds that passed from the beginning of the video to the moment when the individual appeared. In the case of an individual that never appeared during the video, latency was expressed as a missing value. In cases where an individual was already present at the beginning of the video, latency was expressed as 0 s. Since Pied Flycatcher males show no territorial behaviour before finding a suitable breeding site (von Haartman 1955), we consider the latency measurement as an important individual feature of territoriality in the context of paternity protection. Data that were collected subsequently can be categorized within the group of aggressiveness variables.
We first analysed “attack rate”, which consists on the total number of times an individual tried to make physical contact with the decoy per minute, with each attempt being considered as an attack. For example, we considered one attack when the social male arrived at the cage containing the stuffed male, and a second attack when the social male moved away from the cage (more than a distance equivalent to the width of the cage) and then returned to it. We also calculated “peck rate” as the total number of times an individual directed a peck against the decoy per minute. An attack could involve one or more pecks or even no pecks at all and a peck was always acknowledged as an attack.
Finally, we considered “distance equivalent” as a proximity variable representing the percentage of film time that the individual spent at a distance equivalent to the width of the cage or less from the stuffed male. To understand the real distance of the individual from the decoy, the width of the cage (15 cm) that contained the stuffed male was considered as a reference. All the described variables were collected for both males and females.
Statistical analyses
All the analyses were performed using R software (version 3.6.3, R Foundation for Statistical Computing). Three nests were excluded from statistical analyses because the presence of the male during the aggression test was not recorded. For each generalized linear model (GLM, function “glm()”) analysed, a final model was also obtained from the full model with a backward deletion procedure, consisting of removals of variables one by one from the full model when the variance explained did not significantly improve the model (α = 0.05).
Extra-pair paternity and aggressiveness
To test if the frequency of EPP could be influenced by the aggressiveness of the focal male, we constructed a GLM in which the proportion of EPP was represented as dependent variable, and attack rate, peck rate, distance equivalent and male latency as independent variables. In addition, we also included the difference between laying date and video recording date as independent variable to test how the level of male aggressiveness is affected by female fertility. Before running the model, we also checked whether among all the independent variables included in the model there could be a collinearity effect. A Pearson correlation was used for those variables that were normally distributed, while for those variables that did not follow a normal distribution, either a transformation was applied to achieve normality, or the collinearity check was performed using an appropriate univariate model. The collinearity check returned a significant positive correlation between attack rate and peck rate (B = 0.251, p < 0.001, n = 61). Therefore, residuals were calculated correcting attack rate for peck rate and were incorporated into the model as a new variable called "male physical aggression". In this model, following the procedure used in Plaza et al. (2019b), a quasi-binomial distribution was used.
Male and female ornamental traits and aggressiveness
To understand how the level of male aggressiveness is associated with male ornamental traits, three GLMs were run with attack rate, peck rate, and distance equivalent as dependent variables (one dependent variable for each model), and male plumage traits, male age and the difference between laying date and video date as independent variables. As with previous model, we performed a collinearity test among all independent variables prior to running models, but in this case, no significant relationships were found between the independent variables (all p values > 0.05). Hence, following Freckleton (2011), all variables were included in the full models.
Before running the models, Shapiro–Wilk tests were performed to verify the normality of the distribution of dependent variables. As all p values were significant (p < 0.001, n = 61), Poisson distributions were used for the attack rate and peck rate models. Preliminary results of these two models showed, however, the presence of overdispersion, and this was also confirmed by the calculation of a dispersion parameter (φ) estimated using Pearson’s Chi-squared statistic and the degrees of freedom (all values of φ > 46.4). Therefore, to obtain statistically more accurate results from the models and to adjust for overdispersion, quasi-Poisson distributions were used. On the other hand, the Statgraphics software (Version 19.3.03) was used to understand the distribution that best approached the real distribution of the variable “distance equivalent”. After a comparison test of alternative distributions, an exponential distribution was used for distance equivalent model (log-likelihood score = − 142.37).
The same three GLMs were used to analyse the relationship between male aggressiveness and female ornamental traits, because the level of aggressiveness of the male could be influenced by the ornaments of its mate. We used the same dependent variables and female plumage traits, female age, and the difference between laying date and video date as independent variables. Again, the collinearity test showed no significant relationships that could distort the results (all p values > 0.05), so all independent variables were retained in the model (Freckleton 2011). A quasi-Poisson distribution was used for the attack rate and peck rate models (all values of φ > 29.4), while an exponential distribution was used for distance equivalent model (log-likelihood score = − 142.37).
To test if there is a relationship between male traits and the intensity with which a male defends its territory, a GLM with the same independent variables included in the previous models was performed, but with male latency as dependent variable. Female latency was included as an independent variable because during the fertile phase females could affect male mate guarding activity, as during this phase male territoriality may be more directed towards the female than towards the nest. A similar GLM was also run with female traits to see if these characteristics can influence in some way male territoriality. In both models, it was necessary to use an exponential distribution (log-likelihood score = − 437.87).
Results
Extra-pair paternity and aggressiveness
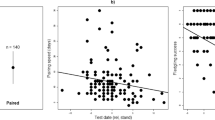
The presence of EPP was found in 21 of 61 broods (34.42%): the paternity tests found 72 of 288 chicks (25.00%) as extra-pair young. EPP nests contained an average of 3.42 ± 1.74 extra-pair young (range 1–6) and 47.62% of these involved one extra-pair male, while 52.38% involved two extra-pair males. The proportion of EPP decreased with an increased male physical aggression (Table 1; Fig. 2; effect size = 44%).
Male ornamental traits and aggressiveness
There were no significant associations between male attack rate or peck rate and male ornamental traits (attack rate: all p values > 0.293, n = 54; peck rate: all p values > 0.375, n = 54), but we found that the time spent in proximity to the intruding male, expressed by male distance equivalent, increased with male UV reflectance (Table 2; Fig. 3; effect size = 30%). The model that analysed the associations between male plumage traits and male latency showed that male latency decreased significantly with male UV reflectance (Table 3; Fig. 4a; effect size = 45%) and increased with female latency (Table 3; Fig. 4b; effect size = 32%).
Female traits and aggressiveness
Male attack rate decreased significantly with an increased extent of the female forehead patch (Table 4; Fig. 5a; effect size = 96%), while male peck rate was negatively associated with the extent of the female extended wing patch (Table 5; Fig. 5b; effect size = 40%). There were no significant associations between male distance equivalent and female ornamental traits (all p values > 0.052, n = 53). There were no associations between male territoriality expressed as male latency and female plumage traits (all p values > 0.103, n = 41).
Associations between: A male attack rate (attacks/min) and female forehead patch (cm2), B male peck rate (pecks/min) and female extended wing patch (cm2). Male attack rate decreases significantly with the extent of female forehead patch (β = − 2.594, p = 0.044), while male peck rate is negatively associated with the extent of the female extended wing patch (β = 0.932, p = 0.003)
Discussion
We found that males that responded with less physical aggression to a territorial intrusion test suffered a higher paternity loss, a crucial confirmation of the territorial aspects of paternity guards. Moreover, the time spent by the male in proximity to the territorial intruders during the fertile phase of their mates, expressed by male distance equivalent, increased with male UV reflectance. Further evidence of the role of male plumage traits in territorial defence was the higher latency of males with reduced UV reflectance when correcting for female latency at the nest. On the other hand, the extent of female forehead and wing patches was negatively related to the aggressiveness of their mates against intruders.
We predicted that more aggressive males should better protect their mates from intruding males and the incidence of EPP should be lower. Our results agree with this hypothesis. We found that the proportion of EPP decreased when the social male showed a higher level of physical aggression against a possible intruder male. This result strongly agrees with a previous study on the same Iberian population. Moreno et al. (2010) showed that males which showed less aggressive behaviour in response to a territorial intrusion playback test suffered a higher paternity loss. Males could be unable to guard their mates efficiently when they have a propensity to avoid confronting territorial intruders near their nest box. Intruding males would thus have more opportunities to approach females in territories owned by less aggressive males. Our first prediction is thus supported by our results, as they suggest that extra-pair paternity is a consequence of a sexual conflict (Arnqvist and Kirkpatrick 2005; Westneat and Stewart 2003) and depends on the social mate’s mate guarding abilities (Moreno et al. 2010), and on the female’s ability to avoid unwanted extra-pair male encounters (Alatalo et al. 1987; Plaza et al. 2019a, b).
Some studies have previously investigated the role of Pied Flycatcher male traits in determining levels of aggressiveness before the start of laying (Moreno et al., 2010), but without considering the role of UV reflectance and the size of white wing patches. Our study considered more aspects of male territoriality and male signalling capacity to give a fuller view on the importance of different male traits. Although Moreno et al. (2010, 2015) found that black males were more aggressive towards intruders, our results did not find an association between male blackness and territorial response to a decoy intruder. This could be explained by the differences in males’ average blackness between studies (our study 89.8 ± 9.4% vs 70.0 ± 2.0% in Moreno et al. (2015)). However, ornaments in birds are supposedly used as honest signals of individual quality and competitive ability (Andersson 1994; Zahavi 1975). Accordingly, our results showed a significant positive association between the time spent by the male in proximity to the territorial intruders and its UV reflectance. Therefore, there seems to be a relationship between male plumage ornamental traits and the levels of male aggressiveness during the female fertile period. This scenario confirms, although only partially, our second prediction, according to which the more aggressive males should present more developed plumage ornaments. Further confirmation is provided by the indication that plumage traits could also be involved in male active control of the territory, as latency decreased with male UV reflectance of white wing patches when correcting for female latency due to mate guarding. In our study, male plumage characteristics were therefore associated both with investment in territorial control and directly with aggression towards identified intruders. Indeed, UV reflectance of dark areas in this species has been suggested to be correlated with male quality (Siitari and Huhta 2002; Sirkiä and Laaksonen 2009). Tentatively we can conclude that our second prediction is supported by the data.
We found a positive association between male and female latency, which is probably the consequence of mate guarding at this breeding stage (Birkhead and Møller 1992; Griffith et al. 2002; Møller and Birkhead 1991; Moreno et al. 2010). If males follow females around to avoid EPCs, one consequence would be that the behaviour of the two mates in general and therefore their behaviour close to the nest during filming would also be associated. Moreover, the morphological traits of the female, and not only the behavioural ones, could be involved in determining the territorial behaviour of the male. In Pied Flycatchers, both sexes have conspicuous white patches on the wings (Lundberg and Alatalo 1992) characterized by UV reflectance. It is thought that the size of the white wing patches in the females is a sign of dominance given its relationship with testosterone levels measured during the incubation period (Cantarero et al. 2015; Moreno et al. 2014). Further evidence suggests that females with large white wing patches breed earlier and show a greater hatching success (Morales et al. 2007). They either arrive earlier at the breeding grounds or are more effective at securing a nest cavity, and they are more efficient incubators (Morales et al. 2007; Plaza et al. 2018). Moreover, previous studies have also focused on the female white forehead patch, highlighting that patch expression in females of Iberian populations is correlated with lower risk of hemoparasite infections and lower levels of stress (Potti and Merino 1996; Moreno and López-Arrabé 2021), and that females with a white forehead patch invest more in reproduction at early ages than non-patched ones (Potti et al. 2013). All these previous studies suggest that the size of white wing and forehead patches are used as a signal of individual quality and social status in female Pied Flycatchers. Therefore, it is also possible that these traits may be used as a signal in inter-sexual interactions.
Thus, we found a trend for males to carry out fewer attacks and pecks if their partner presented larger forehead and wing patches, respectively. These results, however, contradict our third prediction, according to which males should be more aggressive when mated with higher quality females. If female quality and her social status can influence the behaviour of the social partner, it is possible that also intruding males could perceive the same signals and be more likely to give up when the female presents the signs of social dominance (i.e., for the costs of resistance). In this scenario, the risk of losing paternity could be lower for the social partner, so it could reduce its territorial efforts when mated with dominant females. There is little evidence to support the thesis that higher quality females should be better able to evade mate guarding tactics and more capable of selecting extra-pair mates in Iberian Pied Flycatcher populations, while most studies suggest that they are more able to evade or resist unwanted intruder males (Plaza et al. 2019a, b), supporting the sexual conflict hypothesis. Thus, Moreno et al. (2014) found that younger and shorter-winged Pied Flycatcher females engaged in more EPP, showing that these traits may enable them to avoid extra-pair copulations. This result was also confirmed in part by Plaza et al. (2019a, b). We can conclude that our third prediction should be probably adjusted to the effects of female evasion or defence capacity towards potential extra-pair mates in relation to expressed dominance.
To conclude, we found evidence that the proportion of EPP decreases when the social male shows a higher level of physical aggression towards a possible intruder male, which offers support for the basic tenet of the theory of territoriality as paternity defence. In addition, in this species, different aspects of male territorial behaviour are influenced by male ornamental plumage traits. There seems to be a relationship between the level of male aggressiveness and male ornamental traits, as the time spent by the male in proximity to territorial intruders increases with male UV reflectance. This evidence is further supported by the association between male UV reflectance and control of their territories as expressed by latency (although latency is difficult to interpret in this context as it depends on female behaviour due to mate guarding). Finally, male territorial behaviour may be influenced also by female ornamental traits, with males being less aggressive when they are mated with more dominant females which are more capable of avoiding unwanted approaches by extra-pair males.
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Alatalo RV, Gottlander K, Lundberg A (1987) Extra-pair copulations and mate-guarding in the polyterritorial pied flycatcher Ficedula hypoleuca. Behaviour 101:139–155. https://doi.org/10.1163/156853987X00404
Amundsen T (2000) Why are female birds ornamented? Trends Ecol Evol 15:149–155. https://doi.org/10.1016/S0169-5347(99)01800-5
Andersson M (1994) Sexual selection. Princeton University Press, Princeton
Arnqvist G, Kirkpatrick M (2005) The evolution of infidelity in socially monogamous passerines: the strength of direct and indirect selection on extrapair copulation behavior in females. Am Nat 165:S26–S37. https://doi.org/10.1086/429350
Birkhead TR, Møller AP (1992) Sperm competition in birds: evolutionary causes and consequences. Academic Press, London
Birkhead TR, Briskie JV, Lifjeld JT, Slagsvold T (1997) Breeding-cycle patterns of sperm storage in the Pied Flycatcher (Ficedula hypoleuca). Auk 114:792–796
Brown JL (1964) The evolution of diversity in avian territorial systems. Wilson Bull 76:160–169
Brown JL (1969) Territorial behavior and population regulation in birds: a review and re-evaluation. Wilson Bull 81:293–329
Cantarero A, Laaksonen T, Järvistö PE, Gil D, López-Arrabé J, Redondo AJ, Moreno J (2015) Nest defense behaviour and testosterone levels in female pied flycatchers. Ethology 121:946–957. https://doi.org/10.1111/eth.12407
Cantarero A, Laaksonen T, Järvistö PE, López-Arrabé J, Gil D, Moreno J (2017) Testosterone levels in relation to size and UV reflectance of achromatic plumage traits of female pied flycatchers. J Avian Biol 48:243–254. https://doi.org/10.1111/jav.01032
Darwin C (1871) The descent of man and selection in relation to sex. Princeton University Press, Princeton
Eaton MD, Lanyon SM (2003) The ubiquity of avian ultraviolet plumage reflectance. Proc R Soc Biol Sci 270:1721–1726. https://doi.org/10.1098/rspb.2003.2431
Freckleton RP (2011) Dealing with collinearity in behavioural and ecological data: model averaging and the problems of measurement error. Behav Ecol Sociobiol 65:91–101. https://doi.org/10.1007/s00265-010-1045-6
Griffith SC, Owens IPF, Thuman KA (2002) Extra pair paternity in birds: a review of interspecific variation and adaptive function. Mol Ecol 11:2195–2212. https://doi.org/10.1046/j.1365-294X.2002.01613.x
Griffith SC, Parker TH, Olson VA (2006) Melanin- versus carotenoidbased sexual signals: is the difference really so black and red? Anim Behav 71:749–763. https://doi.org/10.1016/j.anbehav.2005.07.016
Hill GE (1999) Mate choice, male quality, and carotenoid-based plumage coloration. In: Adams N, Slotow R (eds) Proceedings of the international ornithological congress, 22nd edn. BirdLife South Africa, Johannesburg, pp 1654–1668
Järvistö PE, Laaksonen T, Calhim S (2013) Forehead patch size predicts the outcome of male–male competition in the pied flycatcher. Ethology 119:662–670. https://doi.org/10.1111/eth.12107
Jawor JM, Breitwisch R (2003) Melanin ornaments, honesty, and sexual selection. Auk 120:249–265. https://doi.org/10.1093/auk/120.2.249
Jenni L, Winkler R (1994) Moult and ageing of European passerines. Academic Press, London
Komdeur J (2001) Mate guarding in the Seychelles warbler is energetically costly and adjusted to paternity risk. Proc R Soc Lond Ser B 268:2103–2111. https://doi.org/10.1098/rspb.2001.1750
Kötél D, Laczi M, Török J, Hegyi G (2016) Mutual ornamentation and the parental behaviour of male and female Collared Flycatchers Ficedula albicollis during incubation. Ibis 158:796–807. https://doi.org/10.1111/ibi.12389
Lambrechts MM, Adriaensen F, Ardia DR et al (2010) The design of artificial nestboxes for the study of secondary hole-nesting birds: a review of methodological inconsistencies and potential biases. Acta Ornithologica 45:1–26. https://doi.org/10.3161/000164510X516047
Lehtonen PK, Laaksonen T, Artemyev AV, Belskii E, Both C, Bures S, Bushuev AV, Krams I, Moreno J, Magi M, Nord A, Potti J, Ravussin PA, Sirkia PM, Sætre GP, Primmer CR (2009a) Geographic patterns of genetic differentiation and plumage colour variation are different in the pied flycatcher (Ficedula hypoleuca). Mol Ecol 18:4463–4476. https://doi.org/10.1111/j.1365-294X.2009.04364.x
Lehtonen PK, Primmer CR, Laaksonen T (2009b) Different traits affect gain of extra-pair paternity and loss of paternity in the pied flycatcher Ficedula hypoleuca. Anim Behav 77:1103–1110. https://doi.org/10.1016/j.anbehav.2009.01.014
Low M (2005) Female resistance and male force: context and patterns of copulations in the New Zealand stitchbird Notiomystis cincta. J Avian Biol 36:436–448. https://doi.org/10.1111/j.0908-8857.2005.03460.x
Low M (2006) The energetic cost of mate guarding is correlated with territorial intrusions in the New Zealand stitchbird. Behav Ecol 12:270–276. https://doi.org/10.1093/beheco/arj025
Lundberg A, Alatalo RV (1992) The pied flycatcher, chap 3. Poyser, London, pp 15–36. https://doi.org/10.5040/9781472597342
Møller AP, Birkhead TR (1991) Frequent copulations and mate guarding as alternative paternity guards in birds: a comparative study. Behaviour 118:170–186
Morales J, Moreno J, Merino S, Sanz JJ, Tomás G, Arriero E, Lobato E, Martínez-de la Puente J (2007) Female ornaments in the pied flycatcher Ficedula hypoleuca: associations with age, health and reproductive success. Ibis 149:245–254. https://doi.org/10.1111/j.1474-919X.2006.00635.x
Morales J, Gordo O, Lobato E, Ippi S, La Martínez-de PJ, Tomás G, Merino S, Moreno J (2014) Female-female competition is influenced by forehead patch expression in pied flycatcher females. Behav Ecol Sociobiol 68:1195–1204. https://doi.org/10.1007/s00265-014-1730-y
Moreno J, López-Arrabé J (2021) The extent of white plumage patches in female Pied Flycatchers Ficedula hypoleuca is negatively associated with corticosterone concentration in partly unpigmented feathers. J Ornithol 162:511–520. https://doi.org/10.1007/s10336-020-01851-z
Moreno J, Martínez JG, Morales J, Lobato E, Merino S, Tomás G, Vásquez RA, Möstl E, Osorno JL (2010) Paternity loss in relation to male age, territorial behaviour and stress in the pied flycatcher. Ethology 116:76–84. https://doi.org/10.1111/j.1439-0310.2009.01716.x
Moreno J, Velando A, Ruiz-de-Castañeda R, González-Braojos S, Cantarero A (2013) Oxidative damage in relation to a female plumage badge: evidence for signalling costs. Acta Ethol 16:65–75. https://doi.org/10.1007/s10211-012-0138-9
Moreno J, Gil D, Cantarero A, López-Arrabé J (2014) Extent of a white plumage patch covaries with testosterone levels in female pied flycatchers Ficedula hypoleuca. J Ornithol 155:639–648. https://doi.org/10.1007/s10336-014-1046-8
Moreno J, Martínez JG, González-Braojos S, Cantarero A, Ruiz-de-Castañeda R, Precioso M, López-Arrabé J (2015) Extra-pair paternity declines with female age and wing length in the pied flycatcher. Ethology 121:501–512. https://doi.org/10.1111/eth.12364
Nakagawa S, Schroeder J, Burke T (2015) Sugar‐free extrapair mating: a comment on Arct et al. Behav Ecol 26:971–972. https://doi.org/10.1093/beheco/arv041
Plaza M, Cantarero A, Cuervo JJ, Moreno J (2018) Female incubation attendance and nest vigilance reflect social signaling capacity: a field experiment. Behav Ecol Sociobiol 72:24. https://doi.org/10.1007/s00265-017-2423-0
Plaza M, Cantarero A, Moreno J (2019a) An experimental increase in female mass during the fertile phase leads to higher levels of extra-pair paternity in pied flycatchers Ficedula hypoleuca. Behav Ecol Sociobiol 73:161. https://doi.org/10.1007/s00265-019-2771-z
Plaza M, Cantarero A, Gil D, Moreno J (2019b) Experimentally flight-impaired females show higher levels of extra-pair paternity in the pied flycatcher Ficedula hypoleuca. Biol Lett 15:20190360. https://doi.org/10.1098/rsbl.2019.0360
Potti J, Merino S (1996) Decreased levels of blood trypanosome infection correlate with female expression of a male secondary sexual trait: implications for sexual selection. Proc R Soc Lond B 263:1199–1204. https://doi.org/10.1098/rspb.1996.0176
Potti J, Montalvo S (1991) Male arrival and female mate choice in pied flycatchers Ficedula hypoleuca in central Spain. Ornis Scand (scand J Ornithol) 1:45–54. https://doi.org/10.2307/3676620
Potti J, Canal D, Serrano D (2013) Lifetime fitness and age-related female ornament signalling: evidence for survival and fecundity selection in the pied flycatcher. J Evol Biol 26:1445–1457. https://doi.org/10.1111/jeb.12145
Rohwer S, Ewald PW (1981) The cost of dominance and advantage of subordination in a badge signaling system. Evolution 35:441–454
Sanz JJ, Potti J, Moreno J, Merino S, Frías O (2003) Climate change and fitness components of a migratory bird breeding in the Mediterranean region. Glob Change Biol 9:461–472. https://doi.org/10.1046/j.1365-2486.2003.00575.x
Senar JC (1999) Plumage coloration as a signal of social status. In: Adams NJ, Slotow RH (eds) Proceedings of the International Ornithological Congress, 22nd edn. Bird Life South Africa, Johannesburg, pp 1669–1686
Siitari H, Huhta E (2002) Individual color variation and male quality in pied flycatchers (Ficedula hypoleuca): a role of ultraviolet reflectance. Behav Ecol 13:737–741. https://doi.org/10.1093/beheco/13.6.737
Silverin B (1998) Territorial behaviour and hormones of pied flycatchers in optimal and suboptimal habitats. Anim Behav 56:811–818. https://doi.org/10.1006/anbe.1998.0823
Sirkiä PM, Laaksonen T (2009) Distinguishing between male and territory quality: females choose multiple traits in the pied flycatcher. Anim Behav 78:1051–1060. https://doi.org/10.1016/j.anbehav.2009.06.022
Sirkiä PM, Adamík P, Artemyev AV, Belskii E, Both C, Bureš S, Burgess M, Bushuev AV, Forsman JT, Grinkov V, Hoffmann D, Järvinen A, Král M, Krams I, Lampe HM, Moreno J, Mägi M, Nord A, Potti J, Ravussin PA, Sokolov L, Laaksonen T (2015) Fecundity selection does not vary along a large geographical cline of trait means in a passerine bird. Biol J Lin Soc 114:808–827. https://doi.org/10.1111/bij.12469
Svensson L (1984) Identification guide to European passerines, 3rd edn. British Trust for Ornithology, Thetford, Stockholm
Von Haartman L (1955) Territory in the pied flycatcher Muscicapa hypoleuca. Ibis 98:460–475
Westneat DF (1994) To guard mates or go forage: conflicting demands affect the paternity of male red-winged blackbirds. Am Nat 144:343–354. https://doi.org/10.1086/285679
Westneat DF, Stewart IRK (2003) Extra-pair paternity in birds: Causes, correlates, and conflict. Annu Rev Ecol Evol Syst 34:365–396. https://doi.org/10.1146/annurev.ecolsys.34.011802.132439
Wingfield JC, Hegner RE Jr, Dufty AM Jr, Ball GF (1990) The ‘challenge hypothesis’: theoretical implications for patterns of testosterone secretion, mating systems, and breeding strategies. Am Nat 136:829–846. https://doi.org/10.1086/285134
Wingfield JC, Lynn SE, Soma KK (2001) Avoiding the ‘costs’ of testosterone: ecological bases of hormone-behavior interactions. Brain Behav Evolut 57:239–251. https://doi.org/10.1159/000047243
Zahavi A (1975) Mate selection: a selection for a handicap. J Theor Biol 53:205–214
Acknowledgements
We thank Javier Cuervo and Irene Saavedra-Garcés for collaboration in the field.
Funding
Open Access funding enabled and organized by Projekt DEAL. Grants IJC2018-035011-I (A.C.), PID2019-109303 GB-I00 (A.C.) and PID2019-106032 GB-I00 (J.M.) funded by MCIN/AEI. https://doi.org/10.13039/501100011033.
Author information
Authors and Affiliations
Contributions
MB contributed to methodology (equal) and writing—original draft (lead). MP contributed to methodology (equal). JM contributed to conceptualization (equal) and funding acquisition (equal). AC contributed to conceptualization (equal), funding acquisition (equal), methodology (equal), and writing—original draft (supporting).
Corresponding author
Ethics declarations
Conflict of interest
None declared.
Ethical approval
We were legally authorized to capture and handle Pied Flycatchers by Consejería de Medio Ambiente de Castilla y León (competent regional authority, protocol number EP/SG/706/2016, according to Royal Decree 53/2013), and by J. Donés, director of “Centro Montes de Valsaín”, to work in the study area. The study was ethically approved by the Ethical Committee of the ‘Consejo Superior de Investigaciones Científicas’ (CSIC).
Additional information
Communicated by F. Bairlein.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Beccardi, M., Plaza, M., Moreno, J. et al. Male aggressiveness during the female fertile phase in relation to extra-pair paternity, plumage ornaments and female traits. J Ornithol 164, 299–310 (2023). https://doi.org/10.1007/s10336-022-02033-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10336-022-02033-9