Abstract
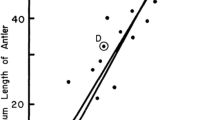
In dimorphic hummingbirds (Trochilidae), females exhibit variation in extent of male-like ornamentation. Female Anna’s Hummingbirds (Calypte anna) show substantial individual variation in the number of pink, male-like gorget feathers. Here, on a sample of nearly 500 female Anna’s Hummingbirds recaptured up to 11 years after banding, we show that gorget size increases substantially when the birds molt from immature into adult plumage. Thereafter, gorget size barely increased with age, meaning that age explains little of the individual variation in number of male-like feathers once a female is adult. A recent study suggested that female polymorphism is widespread in hummingbirds. We reassessed 13 species and show that, relative to adult females, the other “morphs” are actually: immature females; mis-sexed immature or adult males; products of inaccurate field-guide depictions of plumage; an invented morph; subspecific plumage variation; or the conflation of statistical bimodality with polymorphism. We conclude that, although many species of hummingbird show high variation in female plumage, true female polymorphism has only been convincingly demonstrated in perhaps three species (0.9% of hummingbirds). The most striking example is the White-necked Jacobin (Florisuga mellivora). This species appears unique in that adult, breeding females come in two morphs, one that is essentially indistinguishable from the brightly colored males, and the other that is dull and lacks iridescent feathers.
Zusammenfassung
Polymorphismus des Gefieders von Weibchen ist bei Kolibris selten
Bei dimorphen Kolibris (Trochilidae) zeigen die Weibchen unterschiedlich ausgeprägte Zeichnungen, die eigentlich nur bei Männchen vorkommen. Weibchen des Annakolibris (Calypte anna) zeigen starke individuelle Unterschiede in der Anzahl rosafarbener Kehlfedern. An einer Stichprobe von fast 500 Annakolibri-Weibchen, die bis zu 11 Jahre nach der Beringung wiedergefangen wurden, konnten wir zeigen, dass die Größe der farbigen Kehlfedern erheblich zunimmt, wenn die Vögel vom Jungtier-zum Erwachsenengefieder mausern. Später nimmt die Größe mit dem Alter kaum noch zu, was bedeutet, dass bei erwachsenen Weibchen das Alter nur eine geringe Rolle für die individuelle Vielfalt dieser männlichen Kehlfedern spielt. Eine erst kürzlich durchgeführte Untersuchung legt nahe, dass Polymorphismus bei Kolibri-Weibchen weit verbreitet ist. Wir haben 13 Arten neu untersucht und können zeigen, dass in Hinblick auf erwachsene Weibchen die anderen „Morphen“ in Wahrheit dies sind: unreife Weibchen, falsch-geschlechtsbestimmte unreife oder erwachsene Männchen, ein Ergebnis ungenauer Gefiederdarstellungen in Bestimmungsbüchern, erfundene „Morphe“, unspezifische Gefiedervariationen oder Verwechslungen von statistischer Bimodalität mit Polymorphismus. Daraus schließen wir, dass, obwohl viele Kolibriarten große Unterschiede im Gefieder der Weibchen aufweisen, ein echter Polymorphismus der Weibchen nur bei vielleicht drei Arten (0,9 % aller Kolibris) überzeugend nachgewiesen werden konnte. Auffälligstes Beispiel hierfür ist der Weißnackenkolibri (Florisuga mellivora). Diese Art scheint einzigartig unter den Vögeln zu sein, da die erwachsenen, brütenden Weibchen in zwei Morphen vorkommen: eine, die im Wesentlichen nicht von den leuchtend gefärbten Männchen zu unterscheiden ist, und die andere, die matt ist und keine schillernden Federn hat.






Similar content being viewed by others
References
Bleiweiss R (1985) Iridescent polychromatism in a female hummingbird: is it related to feeding strategies? Auk 102:701–713
Bleiweiss R (1992) Widespread polychromatism in female sunangel hummingbirds (Heliangelus: Trochilidae). Biol J Lin Soc 45:291–314
Clark CJ, Rankin D (2019) Subtle, pervasive genetic correlation between the sexes in the evolution of dimorphic hummingbird tail ornaments. Evolution 74:528–543
Clark CJ, Russell SM (2012) Anna’s hummingbird (Calypte anna). In: Poole A (ed) Birds of North America Online. Cornell Lab of Ornithology, Ithaca, NY
Clench M (1976) Possible pitfalls in museum specimen data. North Am Bird Bander 1:20–21
Clyde DP (1972) Anna’s hummingbird in adult male plumage feeds nestling. Condor 74:102
Diamant ES, Falk JJ, Rubenstein DR (2021a) Male-like female morphs in hummingbirds: the evolution of a widespread sex-limited plumage polymorphism. Proce Royal Soc b: Biol Sci 288:20203004
Diamant ES, Falk JJ, Rubenstein DR (2021b) Male-like female morphs in hummingbirds: the evolution of a widespread sex-limited plumage polymorphism. Dryad Dataset, Dryad
Elgar RJ (1978) Dimorphism in a captive female white-necked jacobin. Avic Mag 86:147–149
Falk JJ, Webster MS, Rubenstein DR (2021) Male-like ornamentation in female hummingbirds results from social harassment rather than sexual selection. Curr Biol 31:4381-4387.e4386
Galeotti P, Rubolini D, Dunn PO, fasola M (2003) Colour polymorphism in birds: causes and functions. J Evol Biol 16:635–646
Graves GR (1980) A new species of metaltail hummingbird from northern peru. Wilson Bull 92:1–7
Harris JBC, Ágreda AE, Juiña ME, Freymann BP (2009) Distribution, plumage, and conservation status of the endemic Esmeraldas Woodstar (Chaetocercus berlepschi) of western Ecuador. Wilson J Ornithol 121:227–239
Hartigan JA, Hartigan PM (1985) The dip test of unimodality. Ann Stat 13:70–84
Hawkins GL, Hill GE, Mercadante A (2012) Delayed plumage maturation and delayed reproductive investment in birds. Biol Rev 87:257–274
Hilty SL, Brown WL (1986) Birds of Colombia. Princeton University Press, Princeton, New Jersey
Hogan BG, Stoddard MC (2018) Synchronization of speed, sound and iridescent color in a hummingbird aerial courtship dive. Nat Commun 9:5260
Howell SNG (2003) Hummingbirds of North America the photographic field guide. Princeton University Press, Princeton, NJ
Kimball RT, Ligon JD (1999) Evolution of avian plumage dichromatism from a proximate perspective. Am Nat 154:182–193
Lyon BE, Montgomerie RD (1986) Delayed plumage maturation in passerine birds: reliable signaling by subordinate males? Evolution 40:605–615
Maechler M (2021) Package ‘diptest’. R package version 0.76-0 https://cran.r-project.org/web/packages/diptest/diptest.pdf.
Marques-Santos F, Wischhoff U, Roper JJ, Rodrigues M (2018) Delayed plumage maturation explains differences in breeding performance of Saffron Finches. Emu 118:323–333
Mazariegos L (2000) Hummingbirds of Colombia. Luis A Mazariegos, Bogotá, Colombia
Moore RT (1947) Habits of male hummingbirds near their nests. Wilson Bull 59:21–25
Ortiz-Crespo FI (1972) A new method to separate immature and adult hummingbirds. Auk 89:851–857
Osmond MM, Reudink MW, Germain RR, Marra PP, Nocera JJ, Boag PT, Ratcliffe LM (2013) Relationships between carotenoid-based female plumage and age, reproduction, and mate colour in the American Redstart (Setophaga ruticilla). Can J Zool 91:589–595
Owens IPF, Short RV (1995) Hormonal basis of sexual dimorphism in birds: implications for new theories of sexual selection. Trends Ecol Evol 10:44–47
Parkes KC (1989) Sex ratios based on museum collections: a caution. Colon Waterbirds 12:130–131
Peterson AT, Navarro-Sigüenza AG, Scachetti Pereira R (2004) Detecting errors in biodiversity data based on collectors’ itineraries. Bull Br Ornithol Club 124:143–151
Pyle P (1997) Identification guide to North American birds part 1 Columbidae to Ploceidae. Slate Creek Press, Bolinas, CA
Pyle P (2021) Examination of digital images from Macaulay Library to determine avian molt strategies: a case study on molts and plumages in eight species of North American hummingbirds. BioRχiv. https://doi.org/10.1101/2021.02.03.429637
Pyle P, Engilis A, Kelt D (2015) Manual for ageing and sexing the Landbirds of Bosque Fray Jorge National Park and North-central Chile, with Notes on occurrence and breeding seasonality. Louisiana State University, Baton Rouge
Quesnel VC (1995) The case history of an aberrant Black-throated Mango hummingbird Anthracothorax nigricollis. Bull Br Ornithol Club 115:25–27
Ridgely R, Greenfield PJ (2003) The birds of Ecuador. Cornell University Press, Ithaca, New York
Ridgway R (1911) The birds of North and Middle America. Government Printing Office, Washington DC
Rohwer S (1983) Testing the female mimicry hypothesis of delayed plumage maturation: a comment on procter-gray and holmes. Evolution 37:421–423
Ruschi A (1965) Observações sobre a nidificação, incupação e cuidados com a prole em colibri Coruscans coruscans (Gould), realizado unicamente pela fêmea (Aves. Trochilidae). Bol Mus Biol Mello Leitão 45:1–9
Russell SM, Russell RO (2001) The North American bander’s manual for banding hummingbirds. North American Banding Council, http://www.nabanding.net/manuals/HUMM_MAN.PDF.
Schuchmann KL (1999) Family Trochilidae (Hummingbirds). In: del Hoyo J, Elliott A, Sargatal J (eds) Handbook of the birds of the world. Barn-owls to Hummingbirds, vol 5. Lynx Edicions, Barcelona
Sieburth D, Pyle P (2018) Evidence for a prealternate molt-migration in the Rufous Hummingbird and its implications for the evolution of molts in Apodiformes. Auk 135:495–505
Simpson RK, McGraw KJ (2018) Two ways to display: male hummingbirds show different color-display tactics based on sun orientation. Behav Ecol 29:637–648
Stiles FG (1995) Intraspecific and interspecific variation in moult patterns of some tropical hummingbirds. Auk 112:118–132
Stiles FG, Skutch AF (1989) Birds of Costa Rica. Cornell University Press, Ithaca, NY
Thorogood R, Davies N (2013) Hawk mimicry and the evolution of polymorphic cuckoos. Chinese Birds 4:39–50
Trnka A, Trnka M, Grim T (2015) Do rufous common cuckoo females indeed mimic a predator? An experimental test. Biol J Lin Soc 116:134–143
Valdés-Velásquez A (2003) Taxonomy, phylogeny, and biogeography of the hummingbird genus Thalurania Gould, 1848 (Aves: trochilidae). Ph.D. thesis.
Williamson FSL (1956) The molt and testis cycles of the Anna Hummingbird. Condor 58:342–366
Williamson SL (2002) Hummingbirds of North America. Houghton Mifflin Company, New York
Yanega GM, Pyle P, Geupel GR (1997) The timing and reliability of bill corrugations for ageing hummingbirds. Western Birds 28:13–18
Zimmer JT (1953) Studies of Peruvian birds. No. 63 the hummingbird genera Oreonympha, Schistes, Heliothryx, Loddigesia, Heliomaster, Rhodopis, Thaumastura, Calliphlox, Myrtis, Myrmia, and Acestrura. Am Mus Novit 1604:1–26
Acknowledgements
We thank Gary Stiles, Vitor de Q. Piacentini, Peter Pyle, and Dustin Rubenstein for input, and Rita Colwell for contributing to banding. Banding of Anna’s Hummingbirds complied with the laws of the USA: the techniques in the hummingbird banding manual, the guidelines to the use of wild birds in research, and with permits from the USGS Bird Banding Lab.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Additional information
Communicated by F. Bairlein.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Clark, C.J., Robinson, B. & Remsen, J.V. Female plumage polymorphism is rare in hummingbirds. J Ornithol 163, 735–748 (2022). https://doi.org/10.1007/s10336-022-01975-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10336-022-01975-4