Abstract
Stress in birds has been widely studied through the measurement of heterophil-to-lymphocyte ratio (H/L ratio). In this study we aimed to assess for the first time the potential variation of stress, measured as H/L ratio, associated to geography (between-country variation) and seasonality (between seasons and within the breeding season), as well as the leukocyte profiles, in the threatened Dupont’s Lark (Chersophilus duponti), using samples from Spain and Morocco. Furthermore, we tested whether variation in H/L ratio was associated with variables such as population density, presence of blood parasites and individual body condition. We found that H/L ratio did not vary between countries, but individuals captured during the breeding season showed higher values of H/L compared to non-breeding ones. Neither male density, nor date within the breeding season had an effect on the H/L ratio. In Spain, individuals with higher body condition showed lower H/L ratio regardless of whether they were malaria-infected. In Morocco, malaria-infected individuals showed higher values of H/L ratio than the non-infected birds. Moreover, we found that our average values of H/L ratio in Morocco were within the ranges of other passerines, but not in Spain. Individuals with higher H/L ratios may be more stressed or present higher capability to face stressful situations. Although H/L ratio is a useful and relatively easy way to obtain measure of stress, the impact that the environment might have on stress and the way it is explained by H/L ratio must be addressed carefully. This study provides new insight for this species’ biology and provides useful reference information to test the status and survival of other populations.
Zusammenfassung
Veränderungen im Leukozytenprofil der Dupontlerche ( Chersophilus duponti ) in Spanien und Marokko
Stress bei Vögeln ist durch die Messung des Verhältnisses von Heterophilen zu T-Lymphozyten (H/L-Verhältnis) umfassend untersucht worden. In dieser Studie wollten wir zum ersten Mal die potentiellen Veränderungen des Stresses in Verbindung mit der Geografie (Unterschiede zwischen einzelnen Ländern) und der Saisonalität (zwischen den Jahreszeiten und innerhalb der Brutsaison), gemessen als H/L-Verhältnis, sowie die Leukozytenprofile bei der bedrohten Dupontlerche (Chersophilus duponti) anhand von Proben aus Spanien und Marokko bewerten. Darüber hinaus haben wir untersucht, ob das H/L-Verhältnis mit Variablen wie der Populationsdichte, dem Vorhandensein von Blutparasiten und der individuellen physischen Verfassung zusammenhängt. Wir stellten fest, dass das H/L-Verhältnis zwischen den einzelnen Ländern nicht variierte, aber die während der Brutzeit gefangenen Tiere höhere H/L-Werte aufwiesen als nicht brütende Tiere. Weder die Dichte an Männchen, noch der Zeitpunkt innerhalb der Brutsaison hatten einen Einfluss auf das H/L-Verhältnis. In Spanien wiesen Tiere mit einer besseren physischen Verfassung ein niedrigeres H/L-Verhältnis auf, unabhängig davon, ob sie mit Malaria infiziert waren. In Marokko zeigten malariainfizierte Vögel höhere Werte des H/L-Verhältnisses als nicht infizierte. Außerdem stellten wir fest, dass unsere Durchschnittswerte für das H/L-Verhältnis in Marokko innerhalb der Werte für andere Sperlingsvögel lagen, nicht aber in Spanien. Tiere mit einem höheren H/L-Verhältnis sind möglicherweise gestresster oder besser in der Lage, mit Stresssituationen umzugehen. Obwohl das H/L-Verhältnis ein nützliches und relativ einfach zu beschaffendes Maß für Stress ist, muss der eventuelle Einfluss der Umwelt auf Stress und die Möglichkeiten, diesen durch das H/L-Verhältnis zu erklären, sorgfältig untersucht werden. Diese Untersuchung bietet neue Einblicke in die Biologie dieser Art und liefert nützliche Referenzinformationen für die Bestimmung des aktuellen Status und für das Überleben anderer Populationen.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In many species, the physiological stress of individuals is often the first compensatory response to intrinsic or extrinsic stressors—including those mediated by human activities—aimed to preserve homeostasis and improve the chances of survival and reproduction (Dantzer et al. 2014). Living in risky or stressful environments might chronically activate the physiological stress response of individuals. Therefore, studying and quantifying the physiological responses of individuals to stressors can serve to understand whether individual-level responses impact the viability of populations (Wikelski and Cooke 2006; Cooke et al. 2013) and stress metrics can help to assess the status and future fate of natural populations (Dantzer et al. 2014; McCormick and Romero 2017; Szott et al. 2020).
Levels of health status or stress in birds is often measured through blood parameters, like hormone concentration or leukocyte cell counts. Hormones (e.g. corticosterone) have been widely used to assess physiological stress (Davis et al. 2008; Cockrem 2013; Skwarska 2018). However, their concentrations in blood might change rapidly (1–3 min) following disturbance (short-term stress indicator), which makes its use challenging (Scanes et al. 2020) when working with individuals in the wild, whose control and manipulation is not immediate. Alternatively, the use of haematological parameters, such as the heterophil-to-lymphocyte ratio (hereafter H/L ratio) quantified in blood smears, is considered a reliable measure of long-term physiological stress on birds (Davis and Maney 2018; Gormally and Romero 2020), as they vary after some time (1–4 h depending on the species and the stressor’s nature) since exposure to the stressor and they tend to endure longer than corticosterone levels in blood (Vleck et al. 2000; Davis et al. 2008; Müller et al. 2011; Lebigre et al. 2012; Goessling et al. 2015; Davis and Maney. 2018; Skwarska 2018; Minias 2019; Latimer et al. 2020).
Heterophils are phagocytic leukocytes involved in responses to inflammation, infection, and stress (also known as innate defence). Lymphocytes, on the other side, are the largest cell group of the immune system and are involved in immunoglobulin production and control of the immune defence (adaptive immune defence) (Davis et al. 2008; Banbura et al. 2013; Skwarska 2018). In case of exposure to an external factor, causing an injury or infection, a hormone-mediated alteration of the number of leukocytes in blood is produced, so that the number of heterophils increases to fight the infection (Müller et al. 2011), and the number of lymphocytes decreases to avoid an excessive expenditure of energy (Goessling et al. 2015; Granthon and Williams 2017; Minias 2019). Thus, high values of H/L result from an increase in the production of heterophils and/or a concomitant reduction in lymphocytes as a response to stressors. Among the common stressors identified as having an impact on the H/L ratio of birds are: fear (Campo et al. 2005), handling procedures (Cīrule et al. 2012; Lebigre et al. 2012; Scanes et al. 2020), reproduction costs (Vleck et al. 2000; Ochs and Dawson 2008; Jakubas et al. 2011; Lebigre et al. 2012; Skawarska 2018) or harsh environmental conditions (e.g. low food availability, water deprivation, bad weather conditions; Ruiz et al. 2002; Ochs and Dawson 2008; Plischke et al. 2010; Skawarska 2018). Therefore, differences in H/L ratio may be expected due to population geographic location, year of sampling or time of the year (Norte et al. 2009a). Variation of H/L ratio has been also related to the stress induced by parasites. Parasites usually drain host physiological resources and are therefore expected to influence host leukocyte profiles. The presence of blood parasites, for example, can trigger changes in the levels of leukocytes in birds (Dunn et al. 2013). Several factors can influence the change in the H/L ratio of an individual, such as the degree of virulence of a given parasite, the degree of parasitemia as well as its interaction with the age, sex and body condition (Norte et al. 2009b; Becker et al. 2019). However, the relationship between H/L ratio and blood parasites is yet to be clarified, as non-consistent responses of H/L ratio under parasite infections have been found (Norte et al. 2009b; Skwarska 2018; Becker et al. 2019). For example, some studies have reported that avian malaria parasites (Haemoproteus, Plasmodium) increase H/L ratios (Norte et al. 2009b), whereas others found decreased H/L ratios (Dunn et al. 2013) or even no evidence of such variations (Cornelius et al. 2014; Granthon and Williams 2017).
Interpreting results from H/L ratios can be challenging because baseline values of the species studied should be considered to interpret stress correctly (Davis and Maney 2018). Furthermore, it is not always clear whether a high H/L ratio is related to the effect of a potential stressor or a sign of good health status (see Davis et al. 2008). In the context of blood parasite infections, for example, high H/L ratios have been interpreted both as an indicator of good ability to control and repel parasite infections (Minias 2019), and as evidence of active infections (Davis et al. 2004; Becker et al. 2019). Contradictory results have been also found on the relationship between H/L ratio and birds’ body condition. Ochs and Dawson (2008) showed that female Tree swallows (Tachycineta bicolor) in poorer body condition had higher H/L ratio due to higher levels of stress. On the contrary, Norte et al. (2009c) and Powell et al. (2013) found no significant relationship between the H/L ratio and individual body condition of Great Tits (Parus major) and Noisy Miners (Manorina melanocephala), respectively. Therefore, further research assessing the relationship between the H/L ratio, parasites and the body condition of individuals on different study species under varying conditions (sex and age of individuals, temporality, etc.) is needed to improve our understanding of this topic.
Dupont’s Lark (Chersophilus duponti) is a medium-sized passerine (c. 40 g) classified by the IUCN as Vulnerable globally (BirdLife International 2020), and in Europe (BirdLife International 2020). Its distribution is restricted to natural steppes of Spain and North of Africa (García et al. 2008a; Suárez 2010; García-Antón et al. 2019). In Europe, the species has suffered an annual population decline rate of 3.9% between 2004 and 2015 (Gómez-Catasús et al. 2018). In North Africa, this species also seems to be experiencing a severe decline (BirdLife International 2020). However, there is no updated information on this respect. Moroccan and Iberian populations are long-term isolated (García et al. 2008b), live in different ecological context and show morphological differences (García et al. 2008b; Vögeli et al. 2017; García-Anton et al. 2019). These two populations currently seem to differ in size and conservation status, being the Moroccan population seven times larger than the Iberian one (García et al. 2008a). Habitat loss and fragmentation are the main threats for the species (Tella et al. 2005; Suárez 2010; Pérez-Granados and López-Iborra 2013), which partly explain the concentration of individuals at large connected habitat patches with high food availability (Vögeli et al. 2010; Pérez-Granados and López-Iborra 2013; Gómez-Catasús et al. 2019). Despite the current species’ decline, there is only one study, based on feather corticosterone and stable isotopes values, assessing the physiological variation of the species in relation to environmental conditions (Fairhurst et al. 2013).
The aim of this study is to investigate whether the H/L ratio in Dupont’s Lark is sensitive to both external and internal stressors. First, we examined the potential variation of the H/L ratio between countries, comparing samples from Spain and Morocco to assess whether the countries could be considered as independent units of analysis, and between seasons within each country, to test the hypothesis that stress values are higher during the breeding season, compared to the non-breeding season (Wingfield et al. 1997; Norte et al. 2009a; Pap et al. 2010; Skwarska 2018). During the breeding season, individuals are considered to face higher exposure to environmental stress factors, like the emergence of seasonal parasites, increased energetic expenditure, strong trade-offs between immune function and reproduction, or high conspecific competition. The specific features of Mediterranean shrubsteppes, where the study species inhabits, impose particular sources of stress linked to high predation rates and severe time constraints, such as decreased food availability and increased temperature as the breeding season progresses (see for example Calero-Riestra et al. 2013). Second, we evaluated whether the H/L ratio varied with population density, under the hypothesis that stress increases due to increased social contacts (Jakubas et al. 2011). Finally, we tested whether the H/L ratio was associated with individual’s body condition per se, considering the interaction body condition-malaria infection status, expecting that malaria-infected individuals and/or individuals with poorer body condition would show higher H/L ratio (Skwarska 2018; Becker et al. 2019).
Methods
Study sites
The study was carried out in three different periods: 2005–2006, 2012–2013 and 2017–2018 (Table 1), as part of a long-term monitoring programme started in 2005, on the ecology and conservation of the species in Spain and Morocco.
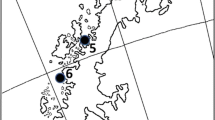
In Spain, we sampled 19 sites in three different regions (same as in García-Antón et al. 2019) varying in orography, climate, vegetation, habitat quality and potential exposure to stressors (Suárez 2010; García-Antón et al. 2019): (1) North Plateau (NP) in the north-west of Spain, with continental Mediterranean climate (locations 1 and 2, Fig. 1); (2) Eastern Corridor (EC) in the southeast of Spain, with arid Mediterranean climate (location 19, Fig. 1); and (3) the Ebro Valley and Iberian Mountains (EV&IM), at the centre of the Spanish distribution of the species and corresponding to the main breeding range of the species in the Iberian Peninsula, with continental Mediterranean climate (locations 3–18, Fig. 1). For a more detailed information see Table S1.
In Morocco, we sampled birds in three populations of Dupont’s Lark living in markedly different habitats, each corresponding to three different sub-regions (see García et al. 2008a): (1) The Ain Bni Mathar population at the High Plateaux (HPlat) (920 m.a.s.l.), located in the province of Jerada (NE Morocco, location 20, Fig. 1), is exposed to a cold semi-arid and hot-summer Mediterranean climate (Verner et al. 2018), with habitat dominated by sparse halfa grass Stipa tenacissima (García et al. 2008a); (2) Rekkam’s population (1400 m.a.s.l.) is located at the Rekkam Plateau (RPlat) (Table S1, location 21, Fig, 1), which shows hot semi-arid and cold desert climate (Verner et al. 2018), with dense halfa grass as the predominant habitat component; and (3) Midelt population (1500 m.a.s.l.), located at the High Molouya (HMol) (Table S1, location 22, Fig. 1), comprising areas of cold desert hot semi-arid and warm-summer Mediterranean climate (Verner et al. 2018) and with sparse halfa grass as the dominant vegetation (García et al. 2008a).
Field and laboratory procedures
Birds were captured during both the breeding and the non-breeding seasons (see Table 1) using tape luring and spring-traps baited with mealworms (Tenebrio molitor larvae). Breeding season lapsed from March 1st to July 10th in Spain (Herranz et al. 1994; Suárez 2010; Pérez-Granados et al. 2017) and from February 1st to June 19th in Morocco (Thévenot et al. 2003, Garcia et al. 2008a). Outside these periods, captures were considered as belonging to the non-breeding season.
Because the capture method used is inherently biased toward males (Laiolo et al. 2007), we excluded the few females (N = 12) and juveniles (N = 2) captured from analyses. A total of 300 Dupont’s Lark males were captured (Table 1), 110 individuals captured during 2005–2006 (43 Spain, 67 Morocco), 35 during 2012–2013 (Spain) and 155 birds during 2017–2018 (Spain) (Table 1).
All birds were ringed to avoid collecting repeated samples over the study period. Blood samples were immediately collected after capture of each individual in the field (see below) and afterwards, the following morphological measurements were taken: wing length, length of the 8th primary (P8) and tail length to the nearest 1 mm with a rule, tarsus length (bent toes) using a digital calliper (accuracy 0.01 mm), and body mass using a digital balance to the nearest 0.1 g. Handling time was reduced to the minimum and never exceeded 30 min.
Blood samples were collected from the jugular or brachial vein, using 0.5 ml insulin syringes and capillary tubes. One drop of blood was used to make a standard smear on a microscope slide, which was fixed in 99% ethylic alcohol for 180 s and air-dried. Afterwards, they were stained with Wright Giemsa stain, and stored at room temperature until microscope inspection (Davis 2005; Banbura et al. 2013). We followed procedures for leukocyte counting described in Campbell (1995) and Carr and Rodak (2010). Slides were visually scanned using an optical microscope (OLYMPUS BX41) under 100 × magnification (using an oil-immersion objective), in a zig-zag pattern, to count the types of leukocytes (heterophils, lymphocytes, eosinophils, basophils and monocytes). A total of 100 leukocytes were identified per slide, noting the proportions of each leukocyte type, and the H/L ratio was calculated by dividing the number of heterophils in these 100 leukocytes by the number of lymphocytes. Only fields of view with relatively uniform distribution of cells were examined. All smears were examined by people with the same training and experience (CPG, SM).
Additionally, we investigated the presence of avian malaria parasites (Plasmodium and Haemoproteus) in samples from both countries using the 2005–2006 sample set. Total genomic DNA was extracted from blood using ammonium acetate and ethanol to precipitate proteins, and purified DNA was diluted to a working concentration of 25 ng/µl. The samples were screened for the presence of Plasmodium and Haemoproteus using a nested polymerase chain reactions (PCR) protocol (Waldenström et al. 2004), designed to amplify a 479-bp fragment of the mitochondrial cytochrome b gene of each parasite genus. We evaluated 2.5 µl of each final reaction on 2% agarose gels stained with ethidium bromide and using 1xTAE buffer. We repeated the protocol three times to confirm negative results.
All birds were captured and handled with the corresponding permissions of both regional and national Spanish and Moroccan authorities, and procedures were approved by the Local Ethical Committee for Animal Experiments of the Autónoma University of Madrid (CEI80-1468-A229, see Ethical Statement).
Statistical analysis
Since both environmental conditions and stressors can vary widely geographically and over time, particularly with respect to time of breeding, we firstly investigated whether both factors had an effect on the H/L ratio (dependent variable) using linear mixed-models (LMM). We used country, season (reproductive/non-reproductive period) and their interaction as fixed factors, and the random effect of sampling year was introduced to account for the potentially high correlation of the data from the same year. Pairwise comparisons among countries and seasons were explored by post-hoc Tukey’s test using the package emmeans (Length 2021) in R software.
After detecting significant variation of H/L ratio considering the interaction between season and country (see below), and as it has been shown how populations differed both morphologically and genetically between Spain and Morocco because of their isolation (García et al 2008b; García-Antón et al. 2018), we focussed further analysis within each country. Furthermore, as we obtained population density estimates only for the breeding season, we restricted further analyses to this period within each country.
A linear regression model (LM) was performed to investigate whether the variation in H/L ratio (response variable) was related to potential stressors in birds’ environment during the breeding season, using population density as a proxy for intraspecific competition (Pérez-Granados and López-Iborra 2015) and date (linear and quadratic term) as potential predictors. Population density estimates in each study site (males per 10 ha) were obtained from García et al. (2008a), Suárez (2010) and Traba et al (2019) (see Table S1). Due to the existing variation in timing of reproduction between countries (Herranz et al. 1994), the variable date was relativized to the start of the season in each country (1st of March for Spain and 1st of February for Morocco), as described in Suárez (2010) and Thévenot et al. (2003). The variable year was included as a fixed factor to control for potential inter-annual variability, since the number of years sampled within each country was not high enough to be considered as a random factor. Lastly, we also included longitude (x) and latitude (y) (UTM coordinates of locations where the animals were captured), as well as the longitude*latitude interaction term to account for potential spatial effects.
We finally explored the relationship between the H/L ratio during the breeding season and body condition and infection status of captured birds using data from 2005 and 2006. We ran a linear regression model using H/L ratio as response variable. The explanatory variables for this model were malaria infection (infected/non-infected), body condition, the interaction between them, and date (linear and quadratic term). As in the previous model, we also included the variable year and longitude (x) and latitude (y), as well as the longitude*latitude interaction term to account for potential spatial correlation. We used the residuals of the regression between body mass and structural size as an index of individual body condition, following the procedure described by Peig and Green (2009). We used wing length as a measure of structural body size because it was the morphological measure that showed the highest correlation with body mass in both countries (Pearson’s test, Spain: R2 = 0.38; p < 0.001; Morocco: R2 = 0.49; p = 0.001).
Final models were chosen based on the deviance statistic for model comparison. Using drop1 function in R (Chambers 1992) we removed those variables that showed the highest p-value, running afterwards a new reduced model and comparing it with the previous one using an Anova (Log-Likelihood ratio Chi-squared test) for the significance of the model. This procedure was carried out until all remaining variables showed a significant effect, and a significant difference between consecutive models tested using Anova function was found.
Lastly, we assessed the variance explained by each model. For the linear mixed model, to know the variance explained by the fixed factors (marginal R2) and from the full model (conditional R2, which considers the variance explained by both the fixed and random factors) we used the R2GLMM function from the package “MuMIn” (Barton 2020) following the procedure described by Nakagawa and Schielzeth (2013). On the other hand, the variance explained by the linear regression models was measured as the adjusted R2.
Statistical analyses were performed using R software (version 3.6.0) (R Core Team 2019). Mixed model was run using the package “lme4” (Bates et al. 2015). Normality of model residuals was visually checked for all models using QQ plots. The H/L ratio was log-transformed to attain normality for the analyses. Continuous predictors were standardized (mean = 0 and SD = 1) within each country. Standarization was obtained using the function decostand of the R package “vegan” (Oksanen et el. 2020).
Results
Leukocyte profiles
The proportions of each white blood cell type and H/L ratio values (mean ± SD) in each study site of both countries are presented in Table 2. In Spain, proportions of lymphocytes at each site ranged from 0.23 to 0.71 (mean ± SD = 0.43 ± 0.20), heterophils from 0.13 to 0.49 (0.32 ± 0.17), eosinophils from 0.01 to 0.40 (0.19 ± 0.16), monocytes from 0.0 to 0.16 (0.06 ± 0.06) and basophils from 0.0 to 0.02 (0.01 ± 0.01). In Morocco, the proportion of lymphocytes at each site ranged from 0.62 to 0.82 (0.73 ± 0.20), heterophils from 0.16 to 0.35 (0.24 ± 0.20) and eosinophils, monocytes and basophils showed an average value of 0.01 (Table 2).
Spatio-temporal variation in H/L ratio
The H/L ratio was significantly higher during the breeding season than during the non-breeding season (χ2 = 10.23; df = 1; p = 0.001) (Fig. 2), and the interaction between season and country had only a marginal effect on H/L ratio (χ2 = 3.07; df = 1; p = 0.079). Tukey’s test showed that differences in H/L ratio between both seasons were significant in Spain, but not in Morocco (Table S2) (Fig. 2). There were no overall differences in H/L ration between both countries (χ2 = 0.41; df = 1; p = 0.519). The full model explained 21.2% of variation in H/L ratio, though the variance of H/L ratio explained by the fixed factors was 10.9% (R2GLMM (m) = 0.109).
Local variation in H/L ratios
In Spain, the H/L ratio varied significantly with the geographic position, as well as with the year of sampling (adjusted R2 = 0.14; Table 3). H/L ratio values decreased with the interaction between longitude and latitude, being the values lower to the north-east (estimate ± SE = − 2.235e-11 ± 1.043e-11; t = − 2.14; p < 0.05). Population density and date were removed from the initital model, as they were not significant in any country. In Morocco, the final model showed that H/L ratio values varied significantly only with longitude (adjusted R2 = 0.14), being the values lower to the east (estimate ± SE = − 1.504e-06 ± 5.598e-07; t = − 2.69; p < 0.05; Table 3).
The influence of malaria infection, which showed a similar prevalence in both countries, and body condition on H/L ratio differed between countries. In Spain, H/L ratio marginally varied with the interaction body condition*malaria (F1,32 = 3.16; p = 0.08), showing a significant variation with longitude (F1,32 = 4.90; p < 0.05) and latitude (F1,32 = 8.01; p < 0.05) (adjusted R2 = 0.32). Non-infected individuals showed an overall independence of H/L values with respect to the body condition, whereas infected individuals showed a decline in H/L values with increasing values of body condition. The infected individuals with high body condition showed lower H/L ratios than non-infected ones (Table 4, Fig. 3), while infected poor-conditon individuals were predicted to show higher H/L ratios than the group of non-infected individuals. Date was removed from the initial model as no significant variation of H/L ratio was detected. Moreover, as we only had data about malaria infection for birds trapped during one year, the variable year was not considered in this case. We did not detect a statistically significant relationship between avian malaria and body condition in Morocco; H/L values in infected individuals did not vary with body condition and were only slightly higher (marginally non-significant: F1,32 = 3.24; p = 0.08) than in uninfected individuals. H/L ratio also increased significantly to the west (F1,32 = 4.77; p < 0.05) (adjusted R2 = 0.16) (Table 4).
Observed values (dots) and predicted values (lines) with a 95% CI of the effect of malaria infection in interaction with body condition on the H/L ratio of Dupont’s Lark males in Spain (a) and Morocco (b) during the breeding season, derived from the linear regression model. Infected individuals are represented in light grey along with a dashed line, and non-infected individuals in black along with a continuous line
Discussion
Our study is the first to provide leukocyte profiles and to examine variation in H/L ratio in the threatened Dupont’s Lark, as well as the link between H/L ratio and different environmental stressors in this species. We found values of H/L ratio similar to those found in other passerines, such as Great tits (Parus major) (average from multiple countries = 0.63 ± 0.13, reviewed in Skwarska 2018), Blue tit (Cyanistes caeruleus) (H/L ratios < 0.20, Banbura et al. 2013), Hooded crow (Corvus cornix) (0.34 ± 0.50; Acquarone et al. 2001), the Red bishop (Euplectes orix) (1 ± 0.78, calculated over different years; Friedl and Edler 2005) or Pied flycatchers (Ficedula hypoleuca) (0.42–0.35 in females; Moreno et al. 2002). Overall, H/L ratios detected in Morocco (average 0.52 ± 0.74) roughly coincide with the average H/L values calculated over different passerine species in a recent review (0.53 ± 0.02; Minias et al. 2019), while the values from Spain (1.11 ± 1.18) are clearly above such average and more similar to the mean reported for ancestral avian lineages (non-passerine species) (1.13 ± 0.08, Minias et al. 2019) or passerines experiencing stress levels (Cornelius et al. 2014). This suggests that Spanish individuals have, on average, higher physiological H/L ratio levels than Moroccan birds.
We observed a temporal variation in this physiological measure of stress. We also observed different local patterns of response in relation with infections status and body condition. The limitations in the knowledge about the relationship between H/L ratio and health status and the unbalanced nature of our dataset implies some caution about the conclusions, but we believe that our results may inform about the health status of populations of this threatened lark. H/L ratio levels were higher during the breeding season compared to the non-breeding season, especially in Spain. We found no relationship between H/L ratio and population densityFinally, when analysing the variation of H/L ratio in relation with individuals’ condition, we did not observe a relationship between avian malaria andbody condition in Morocco. However, in Spain we did show how the presence of malaria affects the H/L ratio of individuals and how this effect could be mitigated with good body condition.
Spatio-temporal variation
Previous studies have demonstrated how Spanish and Moroccan populations of Duponts’ Lark have been genetically disconnected for about 24,000 years (García et al. 2008b). That isolation has resulted not only in a genetical differentiation between countries (García et al. 2008b), but also in morphological variation (García et al. 2008b; García-Antón et al. 2018). Furthermore, habitat structure and composition differ between both study areas (García et al. 2008a; Suárez 2010; García-Antón et al. 2019). All these differences, however, were not substantiated in significant differences in H/L ratio between countries, which suggests similar H/L baseline accounting both in Spain and Morocco.
For instance, as regards temporal variation, we found the same pattern of seasonal change of the H/L ratio in both countries, whith higher H/L ratio during the breeding season than during the non-breeding period. However, this variation was significant only in Spain. In this case, the higher H/L ratio during the breeding season could be related to the territorial behaviour of the species, when there is high competition for finding and defending mates and territories (Garza et al. 2005; Pérez-Granados and López-Iborra 2015). However, this result does not match the lack of relationship between H/L ratio and population density (but see below for further explanations), so it could be also explained by energy demanding constraints during breedingother than intrasexual competition. The relationship between breeding activity and the H/L ratio was already shown by Lebigre et al. (2012), who found that the H/L ratio on Black grouse (Tetrao tetrix) increased during the mating season because of the competition between males and higher demands of energy for reproduction. Our findings are also consistent with those by Pap et al. (2010), who found that the H/L ratio of the Great tit increased during the breeding season (probably caused by an increase of energy demand for parental care and/or a higher presence of parasites, and thus higher levels of infestation), to decrease afterwards (less energetically stressful season and/or absence of parasites in winter). During the non-breeding season, Dupont’s Lark is less territorial and some individuals perform dispersal movements (Suárez et al. 2006). Therefore, competition among males during the non-breeding period might be reduced, compared to the breeding season, which may partly explain the lower H/L ratio found during the non-breeding season. Moreover, we also found that the variance explained by our model was higher when considering the random variable year, which could be due to the potential influence of yearly weather variablility and related food supply on the H/L ratio values (Norte et al. 2009a; Banbura et al. 2013; Fairhurst et al. 2013). Finally, we cannot exclude the role of the high nest predation rates reported in this species (over 80% of nests in some Spanish populations, Herranz et al. 1994; Pérez-Granados et al. 2017; Gómez-Catasús et al. 2021) and other berian ground-nesting shrubsteppe passerines (> 70%, Suarez et al. 1993, Yanes and Suárez 1995) in explaining the observed H/L ratio seasonal differences.
Local variation
In terms of potential environmental stressors, the H/L ratio did not vary significantly in relation to the density of males, contrary to our expectations. High social stress is recognized as a possible cost of reproduction (Jakubas et al. 2011). In several avian species nesting in high density areas, individuals are affected by strong social stress and, subsequently, show an increase in the overall H/L ratio induced by exposure to external stressors (i.e. risk of injury, agonistic interactions, etc.). For example, Vleck et al. (2000) found that H/L ratio in Adélie penguins (Pygoscelis adeliae) decreased after hatching, which suggested a previous stressful situation due to intraspecific competition within the colony and the pressure to defend the clutch against predators. In addition, increased population density has been related to higher infection rate by horizontally transmitted parasites and pathogens, and colonial species often show higher parasitic pressure in large groups (Tella 2002; Brown and Brown 2004) that can affect the stress status of individuals (Raouf et al. 2006). Therefore, it is unlikely that our results actually reflect that a higher male density does not imply a higher overall stress. Conversely, it may indicate (i) a bias in the prevalence/pathogenicity of parasites between populations, (ii) that our density estimates are not the most adequate to reflect social stress, or (iii) that birds from dense areas are actively coping with stressors. First, we found that infections affected the H/L ratio, so the potential differences between populations in this causal factor (or other unmeasured confounders, such as local food availability or local vegetation community Fairhurst et al. 2013) might potentially prevent the detection of more subtle relationships between population density and stress. Second, it is posible that mere the number of males in a certain population does not reflect the true population density. Besides, the spatial scale used to estimate population density might not be the most appropriate. Lastly, it is possible that the populations studied did not reach high enough density values as to have a detectable effect on stress levels.
Once we controlled for the potential spatial (location) and temporal (year) dependency of the data, we analysed the potential influence of infection on the H/L ratio. In relation to the potential variation of H/L ratio with internal stressors we showed how the H/L ratio associated to malaria infection differed between countriesIn Spain, our data suggest a link between H/L ratio and the negative covariation between body condition and infection. This result was similar to the one obtained by Becker et al. (2019) in Dark-eyed juncos (Junco hyemalis), where only infected birds with low body condition showed higher values of H/L ratio. Stressful environments (e.g. resource-poor sites or sites with more pathogenic strains) might reveal negative body condition-infection relationships that are not apparent in more benign environments for hosts. Low values of H/L ratio on individuals with high body condition could be explained by their major resistance against pathogen infections (Jiménez-Peñuela et al. 2019), or the difference in number of parasites needed to cause a significant infection (Marzal et al. 2008). In Morocco, H/L ratio showed no relationship with individual condition. The marginal association with malaria infection could suggest an immune reaction mediated by parasitism (Norte et al. 2009b). Although the different relation of malaria infection,along with body condition,on the H/L ratio is similar to those reported on several bird species (e.g. Cornelius et al. 2014; Granthon and Williams 2017; Skwarska 2018; Becker et al. 2019), results should be interpreted carefully, as the number of infected individuals was quite restricted, which could influence our model performance. Moreover, the potential differences in parasite diversity and pathogenecity within each country (not tested) could also explain these diverging results. In addition, the impossibility of ageing Dupont’s Lark and the small number of females trapped prevented testing the effect of age and sex on H/L ratio variation (Norte et al. 2009b; Jakubas et al. 2011; Isaksson et al. 2013; Dantzer et al. 2014).
Conclusions
In this study, we provide the first values of H/L ratio for populations of the threatened Dupont’s Lark and assessed the potential impact of different stressors on H/L ratio found in the species. Our results highlight season (breeding vs. non-breeding) as an important source of variation of H/L ratio in the species. In addition, we demonstrate the importance of considering individual-level variables, and their interaction, when studying H/L ratio. Our results are based only on adult males, mainly sampled during the breeding season. Therefore, further research considering not only juveniles and females, but also a more evened data for both seasons within each country and for several years. will contribute to a better understanding of how environmental and individual stressors may affect Dupont’s Lark responses to environmental stress (Norte et al. 2009a; Skwarska 2018). Nevertheless, our results might serve as starting point for comparison in future studies aiming to elucidate the health status of threatened Dupont’s Lark populations, as well as for future conservation programmes.
References
Acquarone C, Cucco M, Malacarne G (2001) Short-term effects on body condition and size of immunocompetent organs in the hooded crow. Ital J Zool 68(3):195–199. https://doi.org/10.1080/11250000109356408
Banbura J, Skwarska J, Banbura M, Gladalski M, Holysz M, Kalinski A, Markowski M, Wawrzyniak J, Zielinski P (2013) Spatial and temporal variation in heterophil-to-lymphocyte ratios of nestling passerine birds: comparison of blue tits and great tits. PLoS ONE 8(9):e74226. https://doi.org/10.1371/journal.pone.0074226
Barton K (2020). MuMIn: Multi-Model Inference. R package version 1.43.17. https://CRAN.R-project.org/package=MuMIn. Accessed 24 Jun 2020
Bates D, Mächler M, Bolker B, Walker S (2015) Fitting linear mixed-effects models using lme4. J Stat Softw 67(1):1–48. https://doi.org/10.18637/jss.v067.i01
Becker DJ, Talbott KM, Smiley TM, Clark KL, Sauer PE, Ketterson ED (2019) Leukocyte profiles vary with breeding latitude and infection status in a seasonally sympatric songbird. Anim Migr 6(1):28–40. https://doi.org/10.1515/ami-2019-0004
BirdLife International (2020) Chersophilus duponti. IUCN Red List Threat Spec 2020. https://doi.org/10.2305/IUCN.UK.2020-3.RLTS.T22717380A173711498.en
Brown CR, Brown MB (2004) Empirical measurement of parasite transmission between groups in a colonial bird. Ecology 85(6):1619–1626. https://doi.org/10.1890/03-0206
Calero-Riestra M, García JT, Herranz J, Suárez F (2013) Breeding output and nest predation patterns in steppe-associated Mediterranean birds: the case of the Tawny Pipit Anthus campestris. J Ornithol 154(1):289–298. https://doi.org/10.1007/s10336-012-0893-4
Campbell TW (1995) Avian hematology and cytology, 2nd edn. Iowa State University Press, Iowa
Campo JL, Gil MG, Davila SG (2005) Effects of specific noise and music stimuli on stress and fear levels of laying hens of several breeds. Appl Anim Behav Sci 91(1–2):75–84. https://doi.org/10.1016/j.applanim.2004.08.028
Carr JH, Rodak BF (2010) Atlas de hematologia clinica/clinical hematology atlas. Médica Panamericana, Madrid
Chambers JM (1992) Linear models. Chapter 4 of statistical models in S. Wadsworth & Brooks/Cole, Belmont
Cīrule D, Krama T, Vrublevska J, Rantala MJ, Krams I (2012) A rapid effect of handling on counts of white blood cells in a wintering passerine bird: a more practical measure of stress? J Ornithol 153(1):161–166. https://doi.org/10.1007/s10336-011-0719-9
Cockrem JF (2013) Individual variation in glucocorticoid stress responses in animals. Gen Comp Endocrinol 181:45–58. https://doi.org/10.1016/j.ygcen.2012.11.025
Cooke SJ, Sack L, Franklin CE, Farrell AP, Beardall J, Wikelski M, Chown SL (2013) What is conservation physiology? Perspectives on an increasingly integrated and essential science. Conserv Physiol. https://doi.org/10.1093/conphys/cot001
Cornelius EA, Davis AK, Altizer SA (2014) How important are hemoparasites to migratory songbirds? Evaluating physiological measures and infection status in three neotropical migrants during stopover. Physiol Biochem Zool 87(5):719–728. https://doi.org/10.1086/677541
Dantzer B, Fletcher QE, Boonstra R, Sheriff MJ (2014) Measures of physiological stress: a transparent or opaque window into the status, management and conservation of species? Conserv Physiol. https://doi.org/10.1093/conphys/cou023
Davis AK (2005) Effect of handling time and repeated sampling on avian white blood cell counts. J Field Ornithol 76(4):334–338. https://doi.org/10.1648/0273-8570-76.4.334
Davis AK, Maney DL (2018) The use of glucocorticoid hormones or leucocyte profiles to measure stress in vertebrates: what’s the difference? Methods Ecol Evol 9:1556–1568. https://doi.org/10.1111/2041-210X.13020
Davis AK, Cook KC, Altizer S (2004) Leukocyte profiles in wild house finches with and without mycoplasmal conjunctivitis, a recently emerged bacterial disease. EcoHealth 1(4):362–373. https://doi.org/10.1007/s10393-004-0134-2
Davis AK, Maney DL, Maerz JC (2008) The use of leukocyte profiles to measure stress in vertebrates: a review for ecologists. Funct Ecol 22:760–772. https://doi.org/10.1111/j.1365-2435.2008.01467.x
Dunn JC, Goodman SJ, Benton TG, Hamer KC (2013) Avian blood parasite infection during the non-breeding season: an overlooked issue in declining populations? BMC Ecol 13(1):1–10. https://doi.org/10.1186/1472-6785-13-30
Fairhurst GD, Vögeli M, Serrano D, Delgado A, Tella JL, Bortolotti GR (2013) Can synchronizing feather-based measures of corticosterone and stable isotopes help us better understand habitat-physiology relationships? Oecologia 173:731–743. https://doi.org/10.1007/s00442-013-2678-8
Friedl TW, Edler R (2005) Stress-dependent trade-off between immunological condition and reproductive performance in the polygynous red bishop (Euplectes orix). Evol Ecol 19(3):221–239. https://doi.org/10.1007/s10682-005-0509-z
García JT, Suárez F, Garza V, Justribó JH, Oñate JJ, Hervás I, Calero M, de la Morena ELG (2008a) Assessing the distribution, habitat, and population size of the threatened Dupont’s lark chersophilus duponti in Morocco: lessons for conservation. Oryx 42(4):592–599. https://doi.org/10.1017/S0030605308000653
García JT, Suárez F, Garza V, Calero-Riestra M, Hernández J, Pérez-Tris J (2008b) Genetic and phenotypic variation among geographically isolated populations of the globally threatened Dupont’s lark chersophilus duponti. Mol Phylogenet Evol 46:237–251. https://doi.org/10.1016/j.ympev.2007.06.022
García-Antón A, Garza V, Traba J (2018) Climate, isolation and intraspecific competition affect morphological traits in an endangered steppe bird, the Dupont’s lark chersophilus duponti. Bird Study 65(3):373–384. https://doi.org/10.1080/00063657.2018.1504875
García-Antón A, Garza V, Traba J (2019) Factors affecting Dupont’s Lark distribution and range regression in Spain. PLoS ONE 14(2):e0211549. https://doi.org/10.1371/journal.pone.0219092
Garza V, Suárez F, Herranz J, Traba J, García De La Morena EL, Morales MB, González R, Castañeda M (2005) Home range, territoriality and habitat selection by the Dupont’s lark chersophilus duponti during the breeding and postbreeding periods. Ardeola 52(1):133–146
Goessling JM, Kennedy H, Mendonça MT, Wilson AE (2015) A meta-analysis of plasma corticosterone and heterophil: lymphocyte ratios–is there conservation of physiological stress responses over time? Funct Ecol 29(9):1189–1196. https://doi.org/10.1111/1365-2435.12442
Gómez-Catasús J, Pérez-Granados C, Barrero A, Bota G, Giralt D, López-Iborra GM, Serrano D, Traba J (2018) European population trends and current conservation status of an endangered steppe-bird species: the Dupont’s lark chersophilus duponti. PeerJ 6:e5627. https://doi.org/10.7717/peerj.5627
Gómez-Catasús J, Garza V, Morales MB, Traba J (2019) Hierarchical habitat-use by an endangered steppe bird in fragmented landscapes is associated with large connected patches and high food availability. Sci Rep 9(1):1–12. https://doi.org/10.1038/s41598-019-55467-2
Gómez-Catasús J, Barrero A, Reverter M, Bustillo-de la Rosa D, Pérez-Granados C, Traba J (2021) Landscape features associated to wind farms increase mammalian predator abundance and ground-nest predation. Biodivers Conserv. https://doi.org/10.1007/s10531-021-02212-9
Gormally BMG, Romero LM, Angelier F (2020) What are you actually measuring? A review of techniques that integrate the stress response on distinct time-scales. Funct Ecol 34:2030–2044. https://doi.org/10.1111/1365-2435.13648
Granthon C, Williams DA (2017) Avian malaria, body condition, and blood parameters in four species of songbirds. Wilson J Ornithol 129(3):492–508. https://doi.org/10.1676/16-060.1
Herranz J, Manrique J, Yanes M, Suárez F (1994) The breeding biology of Dupont’s lark, chersophilus duponti. Europe Avocetta 18(2):141–146
Isaksson C, Sepil I, Baramidze V, Sheldon BC (2013) Explaining variance of avian malaria infection in the wild: the importance of host density, habitat, individual life-history and oxidative stress. BMC Ecol 13(1):1–11. https://doi.org/10.1186/1472-6785-13-15
Jakubas D, Wojczulanis-Jakubas K, Glac W (2011) Variation of the reed bunting (Emberiza schoeniclus) body condition and haematological parameters in relation to sex, age and season. Annales Zoologici Fennici. Finn Zool Bot Publ Board. https://doi.org/10.5735/086.048.0405
Jiménez-Peñuela J, Ferraguti M, Martínez-de la Puente J, Soriguer R, Figuerola J (2019) Urbanization and blood parasite infections affect the body condition of wild birds. Sci Total Environ 651:3015–3022. https://doi.org/10.1016/j.scitotenv.2018.10.203
Laiolo P, Vögeli M, Serrano D, Tella JL (2007) Testing acoustic versus physical marking: two complementary methods for individual-based monitoring of elusive species. J Avian Biol 38(6):672–681. https://doi.org/10.1111/j.2007.0908-8857.04006.x
Latimer CE, Smith OM, Taylor JM, Edworthy AB, Owen JP, Snyder WE, Kennedy CM (2020) Landscape context mediates the physiological stress response of birds to farmland diversification. J Appl Ecol 57(4):671–680. https://doi.org/10.1111/1365-2664.13583
Lebigre C, Alatalo RV, Kilpimaa J, Staszewski V, Siitari H (2012) Leucocyte counts variation and measures of male fitness in the lekking black grouse. J Ornithol 153(1):95–102. https://doi.org/10.1007/s10336-011-0701-6
Length, R. V. (2021). Emmeans: estimated marginal means, aka least-squares means. R package version 1.5.5–1
Marzal A, Bensch S, Reviriego M, Balbontin J, De Lope F (2008) Effects of malaria double infection in birds: one plus one is not two. J Evol Biol 21(4):979–987. https://doi.org/10.1111/j.1420-9101.2008.01545.x
McCormick SD, Romero LM (2017) Conservation endocrinology. Bioscience 67(5):429–442. https://doi.org/10.1093/biosci/bix026
Minias P (2019) Evolution of heterophil/lymphocyte ratios in response to ecological and life-history traits: a comparative analysis across the avian tree of life. J Anim Ecol 88(4):554–565. https://doi.org/10.1111/1365-2656.12941
Moreno J, Merino S, Sanz JJ, Arriero E (2002) An indicator of maternal stress is correlated with nestling growth in pied flycatchers Ficedula hypoleuca. Avian Science 2(4):175–182
Müller C, Jenni-Eiermann S, Jenni L (2011) Heterophils/lymphocytes-ratio and circulating corticosterone do not indicate the same stress imposed on Eurasian kestrel nestlings. Funct Ecol 25:566–576. https://doi.org/10.1111/j.1365-2435.2010.01816.x
Nakagawa S, Schielzeth H (2013) A general and simple method for obtaining R2 from generalized linear mixed-effects models. Methods Ecol Evol 4:133–142. https://doi.org/10.1111/j.2041-210x.2012.00261.x
Norte AC, Ramos JA, Sousa JP, Sheldon BC (2009a) Variation of adult great tit Parus major body condition and blood parameters in relation to sex, age, year and season. J Ornithol 150(3):651. https://doi.org/10.1007/s10336-009-0387-1
Norte AC, Araujo PM, Sampaio HL, Sousa JP, Ramos JA (2009b) Haematozoa infections in a great tit parus major population in Central Portugal: relationships with breeding effort and health. Ibis 151(4):677–688. https://doi.org/10.1111/j.1474-919X.2009.00960.x
Norte AC, Sheldon BC, Sousa JP, Ramos JA (2009c) Environmental and genetic variation in body condition and blood profile of great tit Parus major nestlings. J Avian Biol 40(2):157–165. https://doi.org/10.1111/j.1600-048X.2008.04461.x
Ochs CL, Dawson RD (2008) Patterns of variation in leucocyte counts of female tree swallows, tachycineta bicolor: repeatability over time and relationships with condition and costs of reproduction. Comp Biochem Physiol Mol Integr Physiol 150(3):326–331. https://doi.org/10.1016/j.cbpa.2008.04.003
Oksanen J, Blanchet FG, Friendly M, Kindt R, Legendre P, McGlinn D, Minchin PR, O’Hara RB, Simpson GL, Solymos P, Stevens MH (2020) Vegan: community ecology package. R package version 2.5–7
Pap PL, Vágási CI, Tökölyi J, Czirják GÁ, Barta Z (2010) Variation in haematological indices and immune function during the annual cycle in the great tit parus major. Ardea 98(1):105–112. https://doi.org/10.5253/078.098.0113
Peig J, Green AJ (2009) New perspectives for estimating body condition from mass/length data: the scaled mass index as an alternative method. Oikos 118(12):1883–1891. https://doi.org/10.1111/j.1600-0706.2009.17643.x
Pérez-Granados C, López-Iborra GM (2013) Census of breeding birds and population trends of the Dupont’s lark chersophilus duponti in Eastern Spain. Ardeola 60(1):143–150. https://doi.org/10.13157/arla.60.1.2012.143
Pérez-Granados C, López-Iborra GM (2015) Nest failure owing to intraspecific agonistic behaviour in Dupont’s lark chersophilus duponti. Ornithol Sci 14(2):117–121. https://doi.org/10.2326/osj.14.117
Pérez-Granados C, López-Iborra GM, Garza V, Traba J (2017) Breeding biology of the endangered Dupont’s lark chersophilus duponti in two separate Spanish shrub-steppes. Bird Study 64(3):328–338. https://doi.org/10.1080/00063657.2017.1359232
Plischke A, Quillfeldt P, Lubjuhn T, Merino S, Masello JF (2010) Leucocytes in adult burrowing parrots Cyanoliseus patagonus in the wild: variation between contrasting breeding seasons, gender, and individual condition. J Ornithol 151(2):347–354. https://doi.org/10.1007/s10336-009-0461-8
Powell C, Lill A, Johnstone CP (2013) Body condition and chronic stress in urban and rural noisy miners. Open Ornithol J 6(1):25–31. https://doi.org/10.2174/1874453201306010025
R Core Team (2019). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. URL https://www.R-project.org/. Accessed 10 May 2020
Raouf SA, Smith LC, Brown MB, Wingfield JC, Brown CR (2006) Glucocorticoid hormone levels increase with group size and parasite load in cliff swallows. Anim Behav 71(1):39–48. https://doi.org/10.1016/j.anbehav.2005.03.027
Ruiz G, Rosenmann M, Novoa FF, Sabat P (2002) Hematological parameters and stress index in rufous-collared sparrows dwelling in urban environments. The Condor 104(1):162–166. https://doi.org/10.1093/condor/104.1.162
Scanes CG, Hurst K, Thaxton Y, Archer GS, Johnson A (2020) Effect of transportation and shackling on plasma concentrations of corticosterone and heterophil to lymphocyte ratios in market weight male turkeys in a commercial operation. Poult Sci 99(1):546–554. https://doi.org/10.3382/ps/pez485
Skwarska J (2018) Variation of heterophil-to-lymphocyte ratio in the great tit parus major—a review. Acta Ornithol 53(2):103–114. https://doi.org/10.3161/00016454AO2018.53.2.001
Suárez F (2010) La alondra ricotí (Chersophilus duponti). Dirección General para la Biodiversidad. Ministerio de Medio Ambiente y Medio Rural y Marino Medio Rural y Marino, Madrid
Suárez F, Yanes M, Herranz J, Manrique J (1993) Nature reserves and the conservation of Iberian shrubsteppe passerines: the paradox of nest predation. Biol Cons 64(1):77–81
Suárez F, Garcia JT, Sampietro FJ, Garza V (2006) The non-breeding distribution of Dupont’s Lark Chersophilus duponti in Spain. Bird Conserv Int 16(4):317–323. https://doi.org/10.1017/S0959270906000505
Szott ID, Pretorius Y, Ganswindt A, Koyama NF (2020) Physiological stress response of African elephants to wildlife tourism in Madikwe game reserve. S Afr Wildl Res 47(1):34–43. https://doi.org/10.1071/WR19045
Tella JL (2002) The evolutionary transition to coloniality promotes higher blood parasitism in birds. J Evol Biol 15(1):32–41. https://doi.org/10.1046/j.1420-9101.2002.00375.x
Tella JL, Vögeli M, Serrano D, Carrete M (2005) Current status of the threatened Dupont’s lark Chersophilus duponti in Spain: overestimation, decline, and extinction of local populations. Oryx 39(1):90–94. https://doi.org/10.1017/S0030605305000165
Thévenot M, Vernon R, Bergier P (2003) The birds of Morocco: an annotated checklist (No. 20). British Ornithologists Union, Tring
Traba J, Garza V, García-Antón A, Gómez-Catasús J, Zurdo J, Pérez-Granados C, Morales MB, Oñate JJ, Herranz J, Malo J (2019) Criterios para la gestión y conservación de la población española de alondra ricotí Chersophilus duponti. Fundación Biodiversidad, Ministerio para la Transición Ecológica, Madrid
Verner D, Tréguer D, Redwood J, Christensen J, McDonnell R, Elbert C, Konishi Y, Belghazi S (2018) Climate variability, drought, and drought management in morocco’s agricultural sector. World Bank, Washington DC
Vleck CM, Vertalino N, Vleck D, Bucher TL (2000) Stress, corticosterone, and heterophil to lymphocyte ratios in free-living Adélie penguins. The Condor 102(2):392–400. https://doi.org/10.1093/condor/102.2.392
Vögeli M, Serrano D, Pacios F, Tella JL (2010) The relative importance of patch habitat quality and landscape attributes on a declining steppe-bird metapopulation. Biol Cons 143(5):1057–1067. https://doi.org/10.1016/j.biocon.2009.12.040
Vögeli M, Serrano D, Méndez M, Tella JL (2017) Morphological variation in the specialist Dupont’s Lark Chersophilus duponti: geographical clines vs. local ecological determinants. J Ornithol 158:25–38. https://doi.org/10.1007/s10336-016-1383-x
Waldenström J, Bensch S, Hasselquist D, Östman Ö (2004) A new nested polymerase chain reaction method very efficient in detecting plasmodium and haemoproteus infections from avian blood. J Parasitol 90(1):191–194. https://doi.org/10.1645/GE-3221RN
Wikelski M, Cooke SJ (2006) Conservation physiology. Trends Ecol Evol 21(1):38–46. https://doi.org/10.1016/j.tree.2005.10.018
Wingfield JC, Hunt K, Breuner C, Dunlap K, Fowler GS, Freed L, Lepson J (1997) Environmental stress, field endocrinology, and conservation biology. Behavioral approaches to conservation in the wild. Cambridge University Press, London, pp 95–131
Yanes M, Suarez F (1995) Nest predation patterns in ground-nesting passerines on the Iberian Peninsula. Ecography 18(4):423–428. https://doi.org/10.1111/j.1600-0587.1995.tb00145.x
Acknowledgements
This study was partially funded by the authors, the Spanish Ministry of Environment (MMA; J.J. Areces and B. Heredia), the Spanish Agency for International Cooperation (AECI), and the LIFE Ricoti (LIFE15-NAT-ES-000802), supported by the European Comisison. D.B.R. was supported by FPI-UAM fellowship from the Universidad Autónoma de Madrid (UAM), and the collaboration of the Instituto de Investigación en Recursos Cinegéticos (IREC-CSIC-UCLM). We wish to thank the Servicio de Vida Silvestre of Conselleria D’infraestructures, Territori i Medi Ambient (Generalitat Valenciana) and the Moroccan government for ringing permits and supporting our work. We thank V. Garza, A. Ramírez, R. del pozo, E. Juarez, E.L. García de la Morena, F. Suárez, M. Radi, M. Znari and M. Alouí, and the Colectivo Ornitológico Cigüeña Negra for their help during fieldtrips and arrangements. We also thank two anonymous referees whose helpful comments contributed to improve the paper. This work was approved by the Local Ethical Committee for Animal Experiments of the Autónoma University of Madrid (CEI80-1468-A229). All participants accepted to be part of the study.
Funding
Open Access funding provided thanks to the CRUE-CSIC agreement with Springer Nature.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by I. Moore.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Bustillo-de la Rosa, D., Calero-Riestra, M., Pérez-Granados, C. et al. Leukocyte profile variation in Dupont’s Lark (Chersophilus duponti) in Spain and Morocco. J Ornithol 163, 539–551 (2022). https://doi.org/10.1007/s10336-021-01958-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10336-021-01958-x