Abstract
Philopatry and monogamy are conventionally viewed as strategies for improving fitness. Many philopatric and monogamous species have, however, been shown to perform breeding dispersal—an exchange of territory (and often also partner) between two breeding seasons. The adaptiveness of breeding dispersal remains controversial, as data remain scarce and sporadic. For the Northern Goshawk, a typically highly philopatric and monogamous forest raptor, pairs breeding in barren forest landscapes produce fewer fledglings than pairs breeding in more productive landscapes. Using data on Finnish breeding female Goshawks (Accipiter gentilis) during 1999–2016, we tested the hypotheses that: (1) breeding dispersal is more likely at barren territories, (2) dispersing females move to less barren territories, and (3) breeding dispersal improves the survival of young. About 29% of the female Goshawks in our study performed breeding dispersal, which contrasts to philopatry and suggest that site and partner fidelities show large variation within the species’ breeding range. We found no evidence that territorial landscape barrenness (proxy on habitat quality) affects the probability of breeding dispersal. However, females that dispersed upgraded to less barren territories. Nevertheless, there were no subsequent effects of breeding dispersal on reproductive performance, suggesting no obvious difference in the capability of rearing young at either site. Although dispersal events were directed to less barren habitats, we suggest that female dispersal is not driven by the pursue for more prospersous habitats, rather that those females are forced to move, for whatever reason. In addition to other observed reasons such as female–female competition for mates and loss of the original mate, intense logging of mature forests lowering local food availability and restricting nest site availability were likely a partial cause of increased breeding dispersal.
Zusammenfassung
Ausweichen von brutbereiten Weibchen in höherwertige Habitate bei einem brutorttreuen Top-Prädator
Brutorttreue und Monogamie werden üblicherweise als Strategien zur Verbesserung der Fitness angesehen. Für viele philopatrische und monogame Arten wurde jedoch inzwischen nachgewiesen, dass sie zwischen zwei Brutsaisonen ihr Territorium (und oft auch ihren Partner) austauschen. Der Anpassungscharakter dieser Ausbreitung zum Brüten ist nach wie vor umstritten, da nur wenige und sporadische Daten vorliegen. Beim Habicht (Accipiter gentilis), einem normalerweise sehr brutorttreuen und monogamen Wald-Greifvogel, haben in kargen Wäldern brütende Paare weniger Junge als solche in ergiebigeren Habitaten. Anhand von Daten brütender Habichtweibchen aus den Jahren 1999–2016 in Finnland haben wir die Hypothesen getestet, dass (i) Brutausbreitung in kargen Gebieten mit höherer Wahrscheinlichkeit vorkommt, (ii) die Weibchen in weniger karge Gebiete ziehen und (iii) diese Brutausbreitung das Überleben der Jungen verbessert. Etwa 29 % der Habichtweibchen in unserer Studie sind zum Brüten woandershin gezogen, was im Gegensatz zur Philopatrie steht und darauf schließen lässt, dass die Standort- und Partnertreue innerhalb eines Brutgebiets stark variiert. Wir haben keine Hinweise darauf gefunden, dass die Kargheit von Landschaften (für Habitatqualität stehend) die Wahrscheinlichkeit der Ausbreitung von Vögeln zum Brüten beeinflusst. Allerdings zogen diejenigen Weibchen, die zum Brüten wegzogen, in weniger karge Gebiete. Dennoch hatte die Abwanderung keine Auswirkungen auf die Fortpflanzungsleistung, was darauf hindeutet, dass es zwischen beiden Standorten keine offensichtlichen Unterschiede für die Aufzucht von Jungen gibt. Wir vermuten, dass diese Ausbreitung der Weibchen wahrscheinlich kein bewusster Wechsel zu besseren Habitaten ist, sondern dass die Weibchen aus irgendwelchen anderen Gründen zur Abwanderung gezwungen sind. Neben anderen beobachteten Gründen wie z.B. dem Wettbewerb zwischen Weibchen um Partner und dem Verlust des ursprünglichen Partners war wahrscheinlich die intensive Abholzung alter Wälder, die das lokale Nahrungsangebot verringerte und die Verfügbarkeit von Nistplätzen einschränkte, einer der Gründe für die zunehmende Ausbreitung zum Brüten.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The selection of a breeding territory and a partner are among the most central decisions animals make pre-breeding, having direct effects on fitness (Reynolds 1996). In long-lived animals, individuals typically stay with the choice they initially make, and this behaviour has been considered adaptive due to the benefits a familiar territory and/or partner have on fitness (Newton 2010). However, an established territorial adult sometimes changes breeding territory, and often this is also associated with partner replacement. This behaviour is referred to as breeding dispersal and has been broadly reported in the literature, but the current knowledge regarding its causes and consequences remain scarce, and have received little attention (Newton 2010). This is somewhat surprising, as breeding dispersal may have profound implications on the population and its spatial dynamics (Bowler and Benton 2005).
One reason for the lack of research on breeding dispersal is that dispersal events often will go undetected in typical field studies, and is then difficult to separate from mortality, especially if untracked individuals disperse outside the study area (e.g. Cilimburg et al. 2002). Still, an increasing body of literature reports the prevalence of this behaviour, including species earlier considered highly philopatric and monogamous. Several possible drivers of breeding dispersal have been suggested: individual characteristics such as young age (Choudhury 1995; Daniels et al. 2000; Beheler et al. 2003; Blakesley et al. 2006; Cline et al. 2013), being a female (Winkler et al. 2004; Beheler et al. 2003; Pärt and Gustafsson 1989; Calabuig et al. 2008; Cline et al. 2013, cf. Blakesley et al. 2006), low previous breeding success (Pärt and Gustavson 1989; Beheler et al. 2003; Winkler et al. 2004; Calabuig et al. 2008), reproductive failure (Haas 1998; Bradley et al. 1990; Daniels et al. 2000; Blakesley et al. 2006; Newton 2010) as well as spatial or temporal conditions such as sub-optimal habitat quality (Newton 1986, 2010; Forero et al. 1999; Blakesley et al. 2006; Cline et al. 2013). This suggests that various factors may drive breeding dispersal through a range of individual and environmental conditions.
Long-term philopatry and monogamy have been suggested to be more advantageous than breeding dispersal, particularly so in long-lived species (e.g. Bradley et al. 1990). However, long-lived species are also provided with particularly many opportunities for breeding dispersal if they experience disadvantageous conditions at their breeding site. Some studies have shown that breeding dispersal can lead to increased individual breeding success (Forero et al. 1999; Calabuig et al. 2008), or increased habitat quality (Blakesley et al. 2006), but still not necessarily leading to better breeding success than that of philopatric individuals from the same population (Calabuig et al. 2008). Thus, as a potential trade-off, individuals may optimise their current versus future fitness prospects and initiate breeding dispersal as a conditional strategy to avoid remaining in sub-optimal conditions, or may even be circumstantially forced to do so for example due to mate loss or if the original breeding site suffers habitat loss. Clearly, more studies are needed before generalisations can be made.
The Northern Goshawk (Accipiter gentilis) (hereafter Goshawk) is a large raptor known for a high degree of philopatry and monogamy (Krüger and Lindström 2001; Kenward 2006; Rutz et al. 2006). Female Goshawks choose their territory indirectly through their male mates, who themselves compete for territories (Kenward 2006) so that territory quality may reflect individual male quality (Penteriani et al. 2013). However, Goshawks do occasionally show breeding dispersal (Bechard et al. 2006; Kenward 2006; Rutz et al. 2006; Reynolds et al. 2017), but this behaviour is less studied than turnover rates which only reflects a territory where one individual is replaced with a new individual the following year, which is particularly prominent among females (e.g. Selås et al. 2017; Tolvanen et al. 2017). As breeding dispersal is typically associated with replacement of both territory and mate, studies on its causes and subsequent consequences may struggle with separating between the effects of upgrading the partner and upgrading the territory (Choudhury 1995). Naturally, breeding dispersal may be related to neither of these options.
Given that the reproductive success of Goshawks is habitat-dependent (Krüger and Lindström 2001; Krüger 2002; Byholm et al. 2007; Byholm and Kekkonen 2008), the aim of this study is to investigate whether female Goshawks perform breeding dispersal based on their current or future breeding habitat quality, measured as the proportion of forest relative to the proportion of peatland. The relative proportion of these landscape elements has previously been shown to reflect territory quality in Finland: territories with productive forest-dominated habitats host more prey (leading to better breeding performance) than territories where barren bogs (peatland) are more dominating (Byholm and Kekkonen 2008). Finally, since the number of produced goshawk fledglings in the focal study population differs among territories along the forest-peatland gradient as a result of habitat-dependent partial brood loss (Byholm et al. 2007), we also investigate whether egg and nestling survival as measured partial brood loss changes after a breeding dispersal event occurs. We raise three predictions:
-
(1)
Females whose ranges are characterised by low-productive peatland (i.e. less forest) have a greater probability to switch their territory than females breeding in landscapes characterised by forest. If not, this may reflect that females are, for whatever reason, forced to disperse.
-
(2)
Females who switch territory disperse from a low-quality territory to a more productive territory where the proportion of forest is higher.
-
(3)
Females who switch territory succeed in reducing the level of partial brood loss, leading to higher survival of the nestlings, in relation to their previous breeding attempts and annual variation (as seen in other females).
Materials and methods
We used data from 173 breeding events of 55 Goshawk females at 47 territories. The data were collected during 1999–2016 in an area of 4300 km2 around Kristinestad and Närpes in western Finland (62° 00ʹ–62° 55ʹ N, 21° 05ʹ–22° 40ʹ E). The area is dominated by mixed coniferous forest, but peatland and agricultural areas are also common. The area is very flat, with an elevation varying between approximately 1–200 m.a.s.l. Further details of the study area are found in Byholm (2005) and Byholm et al. (2007).
Each year, fieldwork was initiated in early- to mid-May and focussed on registering clutch size by climbing the trees and inspecting the nests. Occupied nests were typically revisited multiple times during the breeding season to ring the chicks and for monitoring brood survival (i.e. “partial brood loss”), by relating the eventual brood size at the time of ringing to the initial clutch size; see Byholm (2005) for details. Shed feathers of the breeding female were commonly found and collected in and around the nest (Selås et al. 2017). As part of the monitoring, breeding adults were trapped at the nest site using a dho-gaza (Zuberogoitia et al. 2008), with a large overrepresentation of females compared to males—which were not the focus our study as they show no indication of breeding dispersal in our study area. Both trapping and DNA analysis of shed feathers were used to identify individuals and hence to detect breeding dispersal. Fieldwork was avoided in the early breeding season to avoid disturbance in the early breeding phase when the breeders are most easily disturbed. In addition, visits to the nest area were kept as short as possible. While trapping of individuals would be the most invasive method, not a single trapped bird aborted their breeding attempt.
Identification of individual females
To collect a sufficiently large sample on female breeding dispersal events, we also identified individual breeding females from multilocus DNA (microsatellites) extracted from the adult female’s moulted feathers around the nest. The feathers were collected during 1999–2006, and the laboratory analyses were completed during 2006–2007 (Ylinen 2008). This is a robust and non-invasive method to distinguish individuals from each other. We identified 35 females from 132 breeding events using feather DNA, 9 females from 20 breeding events from trapping, and 11 females from 21 breeding events from feather DNA complemented with trapping of the bird the same year.
DNA amplification and genotyping of individuals
DNA extraction was performed using the salt extraction method by Aljanabi and Martinez (1997). Slight modifications were made due to predicted lower DNA yield from the feather samples. These included one additional centrifuge stage to remove keratin leftovers (13,000g 8 min), prolonged incubation times (+ 58 °C 48 h 400 rpm or + 58 °C 72 h 0 rpm) and increased amount of proteinase K (10 µl, with mass concentration 20 mg/ml).
Eleven polymorphic microsatellite markers were used for genotyping the birds (Ylinen 2008). The characteristics of these markers are presented in Table S1. The DNA samples were amplified by PCR in a reaction volume of 10 µl using a PTC-100™ Programmable Thermal Controller (MJ Research Inc.). The PCR program included an initial denaturation (94 °C for 3 min), followed by 35 cycles (Step 1 denaturation: 94 °C for 45 s; Step 2 annealing: varied in each loci, see Table S2; Step 3 elongation: 72 °C for 2 min) and a final extension phase (72 °C for 15 min). The PCR was carried out in a reaction volume of 10 µl using three different recipes (Table S3). The amplification products of the 11 microsatellite markers were sequenced using MegaBACE (Amersham Biosciences). The scoring of alleles was performed using the software Fragment Profiler (Amersham Biosciences). Furthermore, the potential scoring errors and null alleles were identified using MICRO-CHECKER (version 2.2.3, Van Oosterhout et al. 2004).
At this stage, microsatellite loci were characterised after removing identical multilocus genotypes from the dataset, so that feathers with unique multilocus genotype were presented only once. GENEPOP (version 3.4, Raymond and Rousset 1995) was then used to test for linkage disequilibrium and deviations from Hardy–Weinberg equilibrium. Allele frequencies, genetic diversity and FIS were determined using FSTAT version 2.9.3.2 (Goudet 1995). IDENTITY version 1.1 (Wagner and Sefc 1999) was used to calculate the probability of identity (PID) for a randomly breeding population, and PID among first-order relatives (PIDsib).
Landscape data
A digital map covering the study area was constructed using ArcGIS 10.5 (ESRI 2017) by combining Multi-source National Forest Inventory data (MS-NFI) with CORINE land cover inventory data (CLC 2006-Finland, 2008) and sediment data for peat deposits > 0.3 m thick as obtained from Geological Survey of Finland (GTK open licence CC BY 4.0, GTK’s Superficial deposits 1:200,000, www.hakku.gtk.fi). Due to different resolution of datasets, spatial data were re-sampled to one common resolution 25 × 25 m. MS-NFI data represent forest composition in 2005 as the amount of wood (m3/ha) and age for the three most common tree species: birch, pine and spruce, and for other broad-leaved tree species (Tomppo et al. 2005). These data were first converted into forest classes according to volume and age, after which the information was combined with the CORINE land cover inventory data following the approach described in Byholm et al. (2020). The prepared map layer was then merged with the rasterised peat deposit polygons resulting in a final map where peat deposits were prioritised over the MS-NFI–CORINE map when overlapping.
For measuring habitat composition in the breeding territories, we used the resulting map to calculate the proportion of forest (mature spruce-, pine- and mixed forest; young forests), clear cuts, peatland, agricultural land, urban areas and water at three different spatial scales (radii 1000 m, 2000 m and 4000 m) around each nest (cf. Byholm et al. 2020). At all three scales, we calculated a multiplicative contrast (the logarithm of a ratio) between the proportion of peat and forest (hereafter called “Barrenness”):
We added 0.05 to the proportion of peatland and forest before applying the natural logarithm to circumvent problems due to zero- or nearly zero values. In general, to avoid outliers, these kinds of procedures can be recommended in analyses of habitats with occasionally low proportions, and it does not notably bias the results (Aebischer et al. 1993). The resulting continuous variable “Barrenness” hence measures the amount of peatland compared to forestland, being zero with an equal proportion of peatland and forest, positive with more peat and negative with more forest in a symmetric manner. Since barren peatlands offer less habitat for most principal goshawk prey species than forests do (Byholm et al. 2007; Byholm and Kekkonen 2008), the food availability for the Goshawk decreases with barrenness.
Statistical analyses
To study whether a higher degree of barrenness of the initial breeding habitat led to higher probability of breeding dispersal, we tested the one-tailed null hypothesis of no such positive association. For this, we constructed a binomial generalised linear model (GLM with binomial error and logit-link function) with a binary response variable (“switched”/“stayed”), explained by the centred continuous variable “Barrenness.c” (with zero mean). We defined breeding dispersal as the first observed events where an individual female was recorded as breeding in one territory 1 year, and in another territory at the next breeding event.
To test whether females who switched territory upgraded their habitat as a result of breeding dispersal (leading to decreased barrenness with larger proportion of forest), we made a before/after comparison of “Barrenness” among the females that switched their territory. For this, we created a linear model with “Barrenness.c” after breeding dispersal as the response variable, and “Barrenness.c” before breeding dispersal as the explanatory variable. The one-tailed null hypothesis was that switching Goshawk females with average initial territories do not achieve reduced barrenness as a result of breeding dispersal (a negative intercept implies an effect of reduced barrenness). The analysis is like a paired samples t-test, but with the extra property that the degree of upgrading/downgrading may depend on the initial condition (the slope may differ from 1). In a few cases, a female was recorded to switch territory more than once. To avoid pseudo-replication we disregarded any breeding events after the second switch. This enables us to compare the situation before and after the first known territory switch. To validate the assumptions for this analysis, we used Shapiro–Wilk tests for testing the null hypothesis that the model residuals were normally distributed.
For analysing how territory switching affects egg and nestling survival (i.e. whether brood loss occurs), we used a binomial mixed effects model with brood size at ringing (k) and clutch size (n) as the response variable(s), hence modelling the binomial probability (or proportion) of survived individuals (p). (In R the syntax requires the input as successes k and failures n–k). Higher expected values in this model imply better egg/fledgling survival, or a lower level of brood loss. The explanatory variables were centralised “Clutch size”, “Switch” and “Barrenness.c”, with an interaction between “Switch” and initial “Barrenness.c”. Since partial brood loss is habitat dependent in the focal goshawk population (Byholm and Kekkonen 2008), the habitat “Barrenness.c” was measured before the switch. We set female identity “Female.ID” and the factor variable “Year” as random effects on the intercept. The effects of “Clutch size”, “Barrenness.c” (expected to be negative), “Switch” and the interaction between “Barrenness.c” and “Switch” (expected to be positive) were tested using one-tailed tests.
The consistent application of one-tailed test is done with clear a priori stated predicted directions of all outcomes of statistical tests, hence not inflating the rate of errors of type 1. In particular, the purpose of our study is to investigate whether poor quality habitat increases the probability of breeding dispersal, whether the dispersing females upgrade their habitat and whether they improve their breeding success. For all the hypotheses tested, an effect in the opposite direction would be difficult to explain in the light of our data, and hence subject to mere speculation. While we reserve hypothesis testing for the stated directions, effects in both directions can be assessed by the reader in terms of the absolute values of the reported t- and Z-statistics, which are the coefficients divided by their standard errors (SE). Values of abs(t) > 2 or abs(Z) > 2 indicate relevant deviations from zero.
For each type of analysis (separate response variable), we fitted the same model separately for the three spatial scales (radii 1000 m, 2000 m and 4000 m). We applied Bonferroni corrections to account for multiple testing at the three spatial scales. Applying the typical 5% risk level (α = 0.05) for statistical significance, the adjusted critical level of null hypothesis rejection applied here was P < 0.0167 (= α/3); and correspondingly P < 0.0333 (= 0.1/3) was used as the critical level for near significance. All statistical analyses were carried out in R version 3.5.1 (R Core Team 2018). For the binomial mixed model, we used package “lme4” (Bates et al. 2015).
Results
DNA amplification and genotyping of individuals
Eighty-eight unique multilocus genotypes were identified within 195 amplified samples. No deviations from Hardy–Weinberg equilibrium were observed (χ2 = 20.53; df = 22; P = 0.55). After Bonferroni correction in a pair-wise comparison of loci, no linkage disequilibrium was detected. Since there was no evidence for non-random associations of alleles at different loci in the study population, all 11 loci can be independently used as genetic markers (Table S1, Table S4). The allele number of a locus varied between 2 and 12, genetic diversity between 0.801 and 0.099, and expected heterozygosity between 0.099 and 0.796 (Table S4). No evidence of inbreeding was observed (FIS = 0.014). The estimated probability that two unrelated birds show the same genotypes was PID 8.87 × 10–9, and for siblings PIDsib 1.72 × 10–3. Thus, the multilocus genotypes determined using 11 polymorphic microsatellite loci provided a reliable tool for individual identification of Goshawks.
Female breeding dispersal
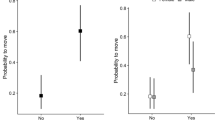
Out of the 55 females, 16 performed breeding dispersal by switching their territory and partner. This corresponds to a rate of 29% (95% CI based on binomial distribution 17.6%–42.9%). Again, the new critical values adjusted for multiple testing was 0.0167 for hypothesis rejection and P < 0.0333 for near significance. The probability for a female to switch territory was not statistically significantly related to initial territorial barrenness at neither 1000 m radius (b = 0.495 ± 0.399 SE, Z = 1.243, P = 0.107); 2000 m radius (b = 0.741 ± 0.473 SE, Z = 1.567, P = 0.059); nor at 4000 m radius (b = 1.217 ± 0.753 SE, Z = 1.616, P = 0.053). At the radius of 1000 m, however, females that switched territory moved to areas with on average significantly lower barrenness, i.e. the habitat quality was upgraded (intercept: − 0.442 ± 0.178 SE, t = –2.485, P = 0.013; Fig. 1). The corresponding estimated effects had the same sign at larger spatial scales, being near significant with 2000 m radius (intercept: − 0.427 ± 0.188 SE, t = − 2.268, P = 0.020) and being non-significant with 4000 m radius (intercept: − 0.087 ± 0.126 SE, t = − 0.693, P = 0.250). In the analysis of barrenness after breeding dispersal, the Shapiro–Wilk tests gave no reason to doubt the assumption of normally distributed residuals at the 1000 m scale (W = 0.936, P = 0.303), where our strongest result was found. However, at the intermediate spatial scale with radius 2000 m, the result was significant, indicating some evidence for non-normality (W = 0.879, P = 0.037), and at the 4000 m scale, the result was near significant (W = 0.904, P = 0.092).
Mean-centred barrenness before and after breeding dispersal in 16 females that had more than 1 territory during the study (circle-symbols represent data points). The black solid line shows the relationship. The vertical grey bar is a 95% confidence interval for the model intercept, showing that on average females switching territory are indeed upgrading. In contrast to the null hypothesis test, the CI is two-tailed with no Bonferroni-correction. With slope 1, this regression would be identical to a paired samples t-test but allowing for another slope corrects for effects of limited availability of high-quality territories. Birds with good (bad) quality territories before have less (more) options to upgrade, even just by chance
There was no statistical evidence for positive effects of breeding dispersal on the probability of partial brood loss at any radius. This was the case for both the main effect of breeding dispersal (variable Switch) and the interaction between breeding dispersal and initial habitat (Table 1).
Discussion
Most Goshawk populations usually have a high level of site-fidelity and thus low level of breeding dispersal (e.g. Krüger and Lindström 2001; Krüger 2005; Kenward 2006; Rutz et al. 2006). In this study, we found a high level of breeding dispersal (29%) in female Goshawks. This high prevalence of breeding dispersal provides some challenge to the view that female Goshawks are highly philopatric and monogamous (Krüger and Lindström 2001; Kenward 2006; Rutz et al. 2006; Reynolds et al. 2017), but rather suggests that the degree of philopatry may show large spatial variation among Goshawk populations throughout their range, here studied at a higher latitude in Finland. Elevated levels of turnover rates have also been found earlier in the Nordics but have been suggested to be mortality driven (Selås et al. 2017; Tolvanen et al. 2017), also providing a contrast to the levels typically found for this species in Europe (Rutz et al. 2006 and references therein). However, a turnover rate does not directly disclose the level of breeding dispersal contributing to its estimate, but sums a range of other factors than territory fidelity. Although being widespread across species, breeding dispersal is often difficult to detect in the field, which leads to a general lack of detailed studies on the topic. We believe the level of breeding dispersal in this study should be representative for the true local rate with our methodological approach of combining trapping of breeders with genetic identification of females, but this does not hinder females to possibly disperse to established males outside the study area. As such cases will remain undetected, the observed breeding dispersal percentage can be viewed as a conservative estimate.
We found no evidence for habitat quality (i.e. landscape productivity) affecting the probability of female breeding dispersal. This contrasts with our prediction and some previous findings in other species (e.g. Forero et al. 1999; Blakesley et al. 2006; Cline et al. 2013), although indicated by Selås et al. (2017). Thus, it may suggest that territorial landscape productivity, here assessed by the proportion of peatland, is not a sufficiently strong or relevant driver itself behind the event of female Goshawks changing their current territory (and mate). Previous studies from other species have shown that events of low breeding success or breeding failure may trigger breeding dispersal (Daniels et al. 2000; Pärt and Gustavson 1989; Haas 1998; Beheler et al. 2003; Winkler et al. 2004; Newton 2010), likely reflecting a negative breeding experience at the particular breeding site. The situation might be opposite of this when starting out in a high-quality territory; Cline et al. (2013) found that dispersal distances were on average shorter for individuals already inhabiting high-quality habitats. In Collared Flycatchers (Ficedula albicollis), previously successful females showed a negative correlation between dispersal distance and subsequent fitness, while this correlation was positive for previously unsuccessful ones (Pärt and Gustavson 1989). The prerequisite for such patterns is that individuals assess their current vs. future prospects based on habitat quality. However, as opposed to earlier observations in Eurasian Sparrowhawk (Accipiter nisus) (Newton 1986), there was no indication that female Goshawks in our study were more prone to disperse after breeding failure. In most cases of breeding failure in our study area, the same pair did not breed the following year, or we lack knowledge about their further activity.
In line with our second prediction, females that dispersed (for whatever reason) moved to higher quality territories (i.e. with a lower barrenness), the result being statistically significant at the 1000 m scale, while being near significant at the 2000 m scale. During the breeding season, the male Goshawk feeds himself, the female and their offspring for long periods (Kenward 2006), and the prevalence of forest-dwelling prey species in close proximity of the nest should be favourable for the breeding Goshawk as a central-place forager (Newton 2010). The finding that females upgraded themselves to more productive territories when dispersing is in line with the previous finding that landscapes with lower degree of barrenness are beneficial for ensuring a good prey base and chick survival (Byholm and Kekkonen 2008). In other species with breeding onset at young age, death of the male partner has been suggested to drive females to disperse to avoid a young partner replacement (Daniels et al. 2000). If this is a driver behind the observed dispersal, it could partly explain the paradox of why in our study a large percentage of females indeed perform breeding dispersal (original male dies), while others remain philopatric even in less-than-optimal habitats (original male survives). But when females, for whatever reason, break their territory and mate bond, they settle in a new territory with potentially more prey that at the one they left (cf. Byholm and Kekkonen 2008).
We could not show any connection between an upgrade in habitat quality and breeding performance, as breeding dispersal was not accompanied by lower brood loss. Habitat quality has earlier been found to affect breeding success in Goshawks (Krüger and Lindström 2001; Krüger 2002; Byholm et al. 2007; Byholm and Kekkonen 2008) and when viewing breeding dispersal as a condition-dependent strategy, our result is somewhat counter-intuitive. When seen in isolation, this may suggest breeding dispersal is a non-adaptive behaviour in Goshawks, unless they are forced to disperse from a disruptive situation with limited options, as a “best of a bad job” rather than a way to maximise fitness. Calabuig et al. (2008) showed in Lesser Kestrels (Falco naumanni) that breeding success increased after breeding dispersal but did still not exceed that of philopatric individuals from the same population. Although we found no relationship between nest site switch and productivity, dispersal may have affected productivity through other traits not accounted for in this study, such as body condition of fledglings or adults, and their subsequent survival after the breeding season.
We did not compare long-term differences on reproductive success such as occupancy rate at the old versus new breeding site, which is often related to habitat quality in birds (Stacey and Legon 1987; Sergio and Newton 2003). It could be difficult to distinguish territory habitat quality from the quality of the male partner (Daniels et al. 2000), but due to intra-specific competition among male Goshawks (Kenward 2006) combined with indications from our study area of lower body condition among males with territories dominated by bog when compared to forest (Byholm et al., unpublished), we believe our estimate of barrenness as a quality measure reflects the quality of the territory holder as well. With the present material we cannot predict what would have happened if the females would have stayed.
Apparently, breeding dispersal may not be an obvious way for female Goshawks to increase their reproductive performance, at least not when performance is measured through nestling survival, as partial brood loss may reflect sub-optimal breeding conditions affecting both parents and offspring. However, we cannot rule out that the switch to a higher quality territory affects the lifetime reproductive success (Krüger 2005) or have possible effects on subsequent survival of fledglings and adults after the breeding season. Still, breeding dispersal occurs in more than a quarter of the females, while the rest show philopatry. Most likely, other factors than habitat quality may be more strongly linked with the breeding dispersal. The decline in long-term occupancy of nest sites in Finland after the 1990s (Hakkarainen et al. 2004; Byholm et al. 2020), mainly driven by intensive logging of mature forests diminishing nest site availability, in combination with concurrent negative prey population trends (e.g. Tornberg et al. 2006), have resulted in a decline of the Finnish Goshawk population (Björklund et al. 2020). We created a subset of our dataset (n = 20) where we had specific information on whether or not any logging occurred before breeding dispersal. Breeding dispersal events as compared between year t and t + 1 matched with a contemporary loss of the breeding forest patch due to logging in (4/20) of the cases. Other cases coincided with the original female being replaced by a new female (5/20; 25%), loss of male partner (3/20; 15%) and nest takeover by competing species (1/20; 5%). In one further case (1/20), breeding dispersal occurred after egg predation in year t, while in the remaining cases (6/20), there was no information available on possible reasons. While the sample size of this subset is small and one have to be cautious in drawing general conclusions, it seems evident that breeding dispersals by female Goshawks are caused by multiple factors. In cases where a female was replaced by a new female, we cannot rule out that the former was expelled by the male partner or a female competitor. In addition to mate loss and the male re-mating with a new female, logging of nest stands commonly coincides with breeding dispersal. As such, some of the breeding dispersal events are thus the result of human caused habitat destruction via logging. Logging has also led to a long-term decline in the forest grouse populations, and low availability of these central prey species affects the Finnish Goshawk population negatively in many ways (Tornberg 1997; Hakkarainen et al. 2004; Byholm et al. 2003; Byholm et al. 2007; Sulkava et al. 2006; Tornberg et al. 2006).
In Northern Europe, Goshawks are strongly dependent on the forest type most attractive to commercial logging, and so are their preferred prey species, with the result of finding themselves in a conflict with the contemporary forestry practices. More generally, the lack of an effect of territorial habitat quality on female breeding dispersal probability together with the observation that multiple other causes coincide with observed dispersal events suggests that the decision to leave an old territory made by female goshawks, and perhaps in birds in general, is often rather a result of a must than a free choice. However, when females for whatever reason conduct breeding dispersal, they succeed on average to find a new breeding site of similar or better quality than the former habitat they left. Given the complexity, we call for more research to resolve pre-dispersal drivers and post-dispersal consequences of breeding dispersal, especially combining these two aspects in same studies.
Data availability
The data used in this study are included in this published article [Electronic Supplementary Material 1].
Change history
14 February 2022
A Correction to this paper has been published: https://doi.org/10.1007/s10336-022-01969-2
References
Aebischer NJ, Robertson PA, Kenward RE (1993) Compositional analysis of habitat use from animal radio-tracking data. Ecology 74:1313–1325
Aljanabi SM, Martinez I (1997) Universal and rapid salt-extraction of high quality genomic DNA for PCR-based techniques. Nucleic Acid Res 25:4692–4693
Bates D, Maechler M, Bolker B, Walker S (2015) Fitting linear mixed-effects models using lme4. J Stat Softw 67:1–48
Bechard MJ, Fairhurst GD, Kaltenecker GS (2006) Occupancy, productivity, turnover, and dispersal of northern goshawks in portions of the northeastern Great Basin. Stud Avian Biol 31:100–108
Beheler AS, Rhodes OE Jr, Weeks HP Jr (2003) Breeding site and mate fidelity in Eastern Phoebes (Sayornis phoebe) in Indiana. Auk 120:990–999
Björklund H, Parkkinen A, Hakkari T, Heikkinen RK, Virkkala R, Lensu A (2020) Predicting valuable forest habitats using an indicator species for biodiversity. Biol Conserv 249:108682
Blakesley JA, Anderson DR, Noon BR (2006) Breeding dispersal in the California spotted owl. Condor 108:71–81
Bowler DE, Benton TG (2005) Causes and consequences of animal dispersal strategies: relating individual behaviour to spatial dynamics. Biol Rev 80:205–225
Bradley JS, Wooller RD, Skira IJ, Serventy DL (1990) The influence of mate retention and divorce upon reproductive success in short-tailed shearwaters Puffinus tenuirostris. J Anim Ecol 59:487–496
Byholm P, Saurola P, Lindén H, Wikman M (2003) Causes of dispersal in Northern Goshawks Accipiter gentilis in Finland. Auk 120:706–716
Byholm P (2005) Site-specific variation in partial brood-loss in northern goshawks. Ann Zool Fenn 42:81–90
Byholm P, Kekkonen M (2008) Food regulate reproduction differently in different habitats: experimental evidence in the Goshawk. Ecology 89:1696–1702
Byholm P, Nikula A, Kentta J, Taivalmäki JP (2007) Interactions between habitat heterogeneity and food affect reproductive output in a top predator. J Anim Ecol 76:392–401
Byholm P, Gunko R, Burgas D, Karell P (2020) Losing your home: temporal changes in forest landscape structure due to timber harvest accelerate Northern goshawk (Accipiter gentilis) nest stand losses. Ornis Fenn 97:1–11
Calabuig G, Ortego J, Cordero PJ, Aparicio JM (2008) Causes, consequences and mechanisms of breeding dispersal in the colonial lesser kestrel, Falco naumanni. Anim Behav 76:1989–1996
Choudhury S (1995) Divorce in birds: a review of the hypotheses. Anim Behav 50:413–429
Cline MH, Strong AM, Sillett TS, Rodenhouse NL, Holmes RT (2013) Correlates and consequences of breeding dispersal in a migratory songbird. Auk 130:742–752
Cilimburg AB, Lindberg MS, Tewksbury JJ, Hejl SJ (2002) Effects of dispersal on survival probability of adult Yellow Warblers (Dendroica petechia). Auk 119:778–789
Daniels SJ, Walters JR (2000) Between-year breeding dispersal in red-cockaded woodpeckers: multiple causes and estimated cost. Ecology 81:2473–2484
Environmental Systems Research Institute (ESRI). 2017: ArcGIS Release 10.5. Redlands, CA
Forero MG, Donázar JA, Blas J, Hiraldo F (1999) Causes and consequences of territory change and breeding dispersal distance in the Black Kite. Ecology 80:1298–1310
Gautschi B, Tenzer I, Müller JP, Schmid B (2000) Isolation and characterization of microsatellite loci in the bearded vulture (Gypaetus barbatus) and cross-amplification in three Old World vulture species. Mol Ecol 9:2155–2234
Goudet J (1995) FSTAT (Version 1.2): A computer program to calculate F-statistics. J Hered 86:485–486
Haas CA (1998) Effects of prior nesting success on site fidelity and breeding dispersal: an experimental approach. Auk 115:929–936
Hakkarainen H, Mykrä S, Tornberg R, Jungell S, Nikula A (2004) Long-term change in territory occupancy pattern of goshawks (Accipiter gentilis). Écoscience 11:399–403
Kenward R (2006) The Goshawk. T & AD Poyser, London
Krüger O (2002) Analysis of nest occupancy and nest reproduction in two sympatric raptors: common buzzard Buteo buteo and goshawk Accipiter gentilis. Ecography 25:523–532
Krüger O (2005) Age at first breeding and fitness in Goshawk Accipiter gentilis. J Anim Ecol 74:266–273
Krüger O, Lindström J (2001) Habitat heterogeneity affects population growth in goshawk Accipiter gentilis. J Anim Ecol 70:171–173
Martinez-Cruz B, David VA, Godoy JA, Negro JJ, O’Brien SJ, Johnson WE (2002) Eighteen polymorphic microsatellite markers for the highly endangered Spanish imperial eagle (Aquila adalberti) and related species. Mol Ecol Notes 2:323–326
Nesje M, Røed KH (2000) Microsatellite DNA markers from the gyrfalcon (Falco rusticolus) and their use in other raptor species. Mol Ecol 9:1433–1449
Newton I (1986) The Sparrowhawk. Poyser, Calton
Newton I (2010) Population ecology of raptors. T & AC Poyser, London
Otterbeck A, Lindén A, Roualét E (2015) Advantage of specialism: reproductive output is related to prey choice in a small raptor. Oecologia 179:129–137
Peck NJ (2000) DNA forensics of raptors and the isolation and characterisation of microsatellite markers in Accipitridae. Dissertation, University of Nottingham
Penteriani V, Rutz C, Kenward R (2013) Hunting behaviour and breeding performance of northern goshawks Accipiter gentilis, in relation to resource availability, sex, age and morphology. Naturwissenschaften 100:935–942
Pärt T, Gustavson L (1989) Breeding dispersal in the collared flycatcher (Ficedula albicollis): possible causes and reproductive consequences. J Anim Ecol 58:305–320
Raymond M, Rousset F (1995) GENEPOP (Version 1.2): Population genetics software for exact tests and ecumenicism. J Hered 86:248–249
Reynolds JD (1996) Animal breeding systems. Trends Ecol Evol 11:68–72
Reynolds RT, Lambert JS, Flather CH, White GC, Bird BJ, Baggett LS, Lambert C, Bayard de Volo S (2017) Long-term demography of the northern goshawk in a variable environment. Wildl Monogr 197:1–40
R Core Team (2018) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria
Rutz C, Bijlsma RG, Marquiss M, Kenward RE (2006) Population limitation in the northern goshawk in Europe: a review with case studies. Stud Avian Biol 31:158–197
Selås V, Kleven O, Steen OF (2017) Female turnover rate differs between two Northern Goshawk Accipiter gentilis nesting areas, as revealed by DNA analysis of moulted feathers. Ibis 159:554–566
Sergio F, Newton I (2003) Occupancy as a measure of territory quality. J Anim Ecol 72:857–865
Stacey PB, Ligon JD (1987) Territory quality and dispersal options in the acorn woodpecker, and a challenge to the habitat-saturation model of cooperative breeding. Am Nat 130:654–676
Sulkava S, Lokki H, Linkola P (2006) The diet of the goshawk Accipiter gentilis during nesting season in Häme (Southern Finland). Suomen Riista 52:85–96
Tolvanen J, Pakanen VM, Valkama J, Tornberg R (2017) Apparent survival, territory turnover and site fidelity rates in Northern Goshawk Accipiter gentilis populations close to the northern range limit. Bird Study 64:168–177
Tomppo E, Haakana M, Katila M, Mäkisara K, Peräsaari J (2009) The multi-source national forest inventory of Finland: methods and results 2005. Working Papers of the Finnish Forest Research Institute 111:1–277. http://urn.fi/URN:ISBN:978-951-40-2151-0
Topinka JR, May B (2004) Development of polymorphic microsatellite loci in the Northern Goshawk (Accipiter gentilis) and cross-amplification in other raptor species. Conserv Genet 5:861–864
Tornberg R (1997) Prey selection of the goshawk Accipiter gentilis during the breeding season: the role of prey profitability and vulnerability. Ornis Fenn 74:15–28
Tornberg R, Korpimaki E, Byholm P (2006) Ecology of the northern goshawk in Fennoscandia. Stud Avian Biol 31:141–157
Van Oosterhout C, Hutchinson WF, Wills DPM, Shipley P (2004) MICROCHECKER: software for identifying and correcting genotyping errors in microsatellite data. Mol Ecol Notes 4:535–538
Wagner HW, Setk KM (1999) IDENTITY 1.0, freeware program for the analysis of microsatellite data. University of Agricultural Sciences, Wien
Winkler DW, Wrege PH, Allen PE, Kast TL, Senesac P, Wasson MF, Llambías PE, Ferretti V, Sullivan PJ (2004) Breeding dispersal and philopatry in the tree swallow. Condor 106:768–776
Ylinen E (2008) Individual identification and territory fidelity of Northern Goshawk Accipiter gentilis. Master thesis, University of Joensuu (in Finnish with English summary)
Zuberogoitia I, Martínez JE, Martínez JA, Zabala J, Calvo JF, Azkona A, Pagán I (2008) The Dho-Gaza and mist net with Eurasian eagle-owl (Bubo bubo) lure: effectiveness in capturing thirteen species of European raptors. J Raptor Res 42:48–51
Acknowledgements
We are grateful to Jukka-Pekka Taivalmäki for his yearlong commitment to assisting with catching breeding Goshawks. The Editor and three anonymous referees provided highly valuable comments on our manuscript.
Funding
AO was funded by Waldemar von Frenckell Foundation, RG was funded by Kone Foundation (Grant no. 201800932). Open Access funding provided by Novia University of Applied Sciences.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by O. Krüger.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this article was revised, in order to correct the affiliation for the co-author Eeva Ylinen.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Otterbeck, A., Lindén, A., Gunko, R. et al. Female breeding dispersal to higher quality habitats in a philopatric top predator. J Ornithol 163, 83–92 (2022). https://doi.org/10.1007/s10336-021-01943-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10336-021-01943-4