Abstract
Natal dispersal is a well-studied phenomenon that can be divided into three stages: (1) starting from an area, (2) wandering to another area and (3) either settling in that area to breed or merely temporarily stopping there before continuing to wander. During the third phase, we can distinguish breeders from non-breeders, which may show similar or different patterns of space use. Breeding Common Ravens are territorial year-round; non-breeders are highly vagrant but may gather at food sources and night roosts for varying lengths of time. In contrast to the wandering phase, little is known about the space use of ravens at such “stop” sites. Here, we used radio telemetry to investigate the space use of 21 non-breeding ravens in the Austrian Alps during a stop stage at an anthropogenic food source. The tagged ravens were present in 69.2 % of the relocation attempts. They used only 27.0 ha (range 6.7–59.7 ha, 95 % utilisation distribution) of the study area, and their activity ranges strongly overlapped with each other. However, within this shared space, sub/adult non-breeders could be found at individually distinct locations, while juveniles showed similar spatial distributions. These results, combined with reported long-distance movements, underline the high behavioural flexibility of non-breeding ravens, which may be a reason for their success in very different habitats throughout the Northern Hemisphere.
Zusammenfassung
Raumnutzung nicht-brütender Kolkraben nahe einer dauerhaften menschlichen Nahrungsquelle
Ein gut untersuchtes Phänomen ist die Abwanderung von Jungtieren. Es lassen sich drei Phasen beobachten: (1) Das Verlassen eines Gebietes (2) Das Einwandern in ein neues Gebiet (3) Ein Ansiedeln, um Junge aufzuziehen oder ein kurzer Aufenthalt vor dem Weiterziehen. Hinsichtlich der Raumnutzung unterscheiden sich in der dritten Phase Brüter und Nichtbrüter mehr oder weniger stark: Während sich beim Kolkraben die Brüter das ganze Jahr über streng territorial verhalten, wandern Nichtbrüter umher, um sich an ergiebigen Nahrungsquellen und Schlafplätzen vorübergehend niederzulassen. Über die Raumnutzung von Nichtbrütern ist in diesem Zusammenhang noch recht wenig bekannt. Mit Hilfe von Radiotelemetrie untersuchten wir dies bei 21 nicht-brütenden Kolkraben in den Österreichischen Alpen in der Umgebung einer reichhaltigen menschlichen Nahrungsquelle. Die besenderten Raben hielten sich in 69.2 % der Fälle im Studiengebiet auf. Jedes dieser Individuen nutzte hierbei lediglich eine Fläche von durchschnittlich 27 Hektar (reichend von 6.7 bis 59.7 Hektar; bei 95 % Raumnutzungsverteilung), welche sich untereinander stark überschnitten. Während subadulte sowie adulte Raben auch noch innerhalb dieses gemeinsam genutzten Areals individuelle Ortspräferenzen zeigten, hielten sich juvenile in demselben Bereich auf. Die Kombination dieser Ergebnisse mit den aus der Literatur bekannten Wanderungen über weite Strecken unterstreicht das flexible Verhaltensrepertoire nicht-brütender Kolkraben. Dies könnte auch ein Grund für deren erfolgreiche Verbreitung in den unterschiedlichsten Lebensräumen der Nordhalbkugel zu sein.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Natal dispersal, defined as the movement of animals from their natal site to their breeding location(s), involves three successive behavioural stages: (1) “start”, when an individual leaves its place of birth; (2) “transience” or “wandering”, when it explores other areas before settling in a new area; and (3) “stop”, when it either stays in a breeding territory or resides only temporarily at a settlement during the dispersal process (Bowler and Benton 2005; Delgado and Penteriani 2008; Clobert et al. 2009). Travelling across unknown areas can involve high costs and mortality (e.g. Waser et al. 1994; Alberts and Altmann 1995), but efficiency can increase as spatial memory improves and as the individual learns about its environment (Saarenmaa et al. 1988; Vuilleumier and Perrin 2006). When an animal temporarily settles in an area during dispersal, it may reflect the transition from exploratory strategies with incomplete environmental information to a more specific use of spatial resources by increasing the value of familiar space (Delgado et al. 2009).
Individuals can be characterised as “breeders” and “non-breeders” according to their social status; these may show substantial differences in their behaviour, especially when breeders defend their territories. In some species, non-breeders may live secretly in a breeder’s territory (Smith 1978; Arcese 1987); in others, the social classes show a clear discrepancy in habitat use (Campioni et al. 2012). In either case, non-breeders are relatively difficult to study and, compared to breeders, little is known about the behaviour and intraspecific interactions of non-breeders or floaters (Smith 1978; Penteriani and Delgado 2011). This is surprising, since it is important to investigate the behaviour and ecological role of non-breeders in order to understand the structure and dynamics of a population and the evolution of the behavioural differences between social classes (Campioni et al. 2010). Here, we define non-breeders as all non-breeding individuals, in contrast to the frequently used term “floaters”, which includes only a fraction of sexually mature non-breeders.
The Common Raven (Corvus corax) is a territorial species that exhibits a variety of behavioural differences between the social classes. Breeding pairs are long-term monogamous and defend a territory, often larger than 10 km2, year-round (Heinrich 1989; Rösner and Selva 2005). Young ravens join non-breeder groups for foraging and roosting after they become independent from their parents during their first summer (Haffer and Kirchner 1993; Ratcliffe 1997; Wright et al. 2003). These non-breeder groups can be highly vagrant (Heinrich et al. 1994) or develop preferences for certain foraging techniques (Dall and Wright 2009) and/or foraging sites (Braun et al. 2012). To change status from a non-breeder to a breeder, individuals have to become sexually mature, find a partner and be able to defend a territory, which does not occur before the age of 3–4 years (Ratcliffe 1997; Webb et al. 2009); indeed, this may take 10 years or more in a satiated population (Loretto and Bugnyar, unpublished data).
Results from several studies on the space use of breeding and non-breeding ravens emphasise that breeders share little space with neighbouring breeding pairs and show strong site fidelity, while the gregarious non-breeders move widely and show intersections of home ranges, especially at rich food sources (Heinrich et al. 1994; Marzluff and Neatherlin 2006; Webb et al. 2012; Scarpignato and George 2013). Large numbers of ravens are attracted by ephemeral food sources such as carcasses or road kill (Heinrich 1989; Marzluff et al. 1996; Stahler et al. 2002) and even more by anthropogenic point subsidies such as farms, landfills, and game parks (Huber 1991; Drack and Kotrschal 1995; Boarman et al. 2006; Webb et al. 2011; Baltensperger et al. 2013; Bijlsma and Seldam 2013). Non-breeders sometime use permanent food sources on a regular basis, which may cause a “stop” stage during dispersal. In such a situation, socialised subgroups, social bonds and rank hierarchies can emerge (Braun et al. 2012; Braun and Bugnyar 2012). However, it is unknown how ravens use the space at such a stop stage. Do they permanently stay next to the food source (e.g. all day, or even all year), leading to a high density of individuals in a small area and potential social challenges, or do they spread out over a larger area after feeding and regularly return to the food source only to feed?
In the study reported in the present paper, we focused on the space use of non-breeding ravens at a “stop” stage, based on data gathered with radio telemetry. We predicted that (1) non-breeders would be restricted to a certain area around the food source which was also used (but not defended) by territorial breeding pairs. We further predicted that (2) this would result in very high overlap between the non-breeders’ activity ranges. Finally, we also predicted, based on recent literature (Roth et al. 2004; Webb et al. 2011, 2012), that (3) we would not find differences in space use between males and females. However, we expected age class and experience to have an effect. Low-ranking and less experienced juveniles might be less competitive and less efficient at finding the best locations to forage, cache food and roost, which could result in more space being used due to increased searching behaviour. In contrast, experienced sub/adults might show stronger site preferences and thus more restricted space use.
Methods
Field site and animals
We conducted our study from September 2011 to January 2014 in the inner Almtal, a narrow valley in the northern Austrian Alps, which has its lowest elevation at around 500 m above sea level and is surrounded by mountain peaks up to 2515 m high. The main focus in this area was on the Cumberland Wildpark (47°48′14″N, 13°56′55″E, Fig. 1), a local game park where wild ravens scrounge food from the captive animals throughout the year. This rich resource can lead to a higher density of breeding pairs than in other areas, with 6.8 nests per 100 km2 observed in 1993 (Drack and Kotrschal 1995). During a long period of persecution, ravens almost became extinct in central Europe in the nineteenth and twentieth centuries (Haffer and Kirchner 1993), but they are now protected year-round by an EU bird directive, and the size of the raven population appears to have stabilized in the Alps (personal observation). At our field site, around 250 ravens have been marked individually with patagial wing tags (Caffrey 2000) and coloured leg rings since 2008. To perform the marking, the birds were caught in the game park using three drop-in traps built after Stiehl (1978) without a compartment for decoy birds; instead, we only baited with meat and bread. Traps were checked at least twice a day (usually in the morning and before sunset) to enable birds to return to their night roost after marking. Caught ravens were handled and released as quickly as possible (after approximately 15 min), and care was taken to ensure that the head and eyes of each raven were covered during the whole procedure by a soft piece of cloth to prevent them from learning about human facial features (compare Marzluff et al. 2010; Cornell et al. 2012). While marking, we collected blood samples for sexing (following the procedure of Griffiths et al. 1998). Individuals were categorised as juveniles (≤1 year old) and sub/adults (>1 year old). Note that subadults (i.e. those in their second and third years) can be distinguished from adults based on their mouth and feather colouration (Heinrich and Marzluff 1992; Heinrich 1994), but since both subadult and adult individuals in this study were exclusively non-breeders, we decided to combine them into one category.
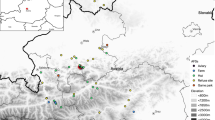
The study area in the northern Austrian Alps; the relocations of 21 Common Ravens are shown (n = 2184) by black x symbols; the main food sources (enclosures of wild boars, bears and wolves) are indicated by the light green area, the detection range for radio signals (limited by mountains) by the purple dashed line, the night roost by the pink dashed ellipse and the game park by the dark green line. The box in the upper right of the figure shows Austria; this has a small rectangle in the centre which indicates the area corresponding to the main map and a black dot showing the location of the capital, Vienna (colour figure online)
The most attractive time for ravens to forage in the game park is between 8 and 10 am, when the captive bears, wolves and wild boars are fed (on average one bucket of meat for wolves, two buckets of bread, meat and vegetables for bears and three to six buckets of chow, bread, vegetables, kitchen leftovers and meat for wild boars). Ravens co-feed and carry away large amounts of food to store it in caches. Depending on the season, 15 (summer)–120 (winter) non-breeders congregate at these feeding sessions and at adjacent night roosts. In addition, there are about 7–10 breeding pairs that occupy nearby territories (Drack and Kotrschal 1995) and use the game park feeding sessions at varying frequencies (Loretto and Bugnyar, unpublished data). Non-breeders can also be distinguished from territorial breeders outside of the breeding period by their submissiveness and suppressed self-aggrandising behaviours, as well as the fact that they do not consistently leave in the direction of a known territory (see Heinrich 1988). During the study period, the presence of marked individuals at the morning feeding sessions of bears, wolves and wild boars was monitored on 578 days (66.6 % of all days during that period), homogeneously distributed over the 3 years.
Radio tracking
In 2011 and 2012 we outfitted 11 individuals (3 males and 8 females with body weights ranging from 975 to 1220 g) with backpack-mounted radio transmitters (Buehler et al. 1995) that weighed 22.5 g and had an estimated lifespan of 24 months (Biotrack Ltd, Wareham, UK; http://www.biotrack.co.uk). These individuals were 2–7 years old, already marked, and had regularly been observed in the game park during previous months; accordingly, they were classified as experienced non-breeders (Table 1).
In the autumn of 2013, we outfitted 14 juvenile ravens (with body weights ranging from 930 to 1235 g) with radio transmitters (Kenward 2001) that weighed 15.8 g and had an expected lifespan of 12 months (Biotrack Ltd). Since raven mortality may be high during their first year (e.g. 47 % survival was reported from the western Mojave Desert, CA, USA; Webb et al. 2004), we wanted to keep any impact of tagging as low as possible and so we chose to use lighter, tail-mounted radio tags (Kenward 2001), which have the additional advantage that ravens lose them when moulting. Six juveniles (2 males, 4 females) out of 14 were caught individually in the drop-in traps during October and hence were of unknown origin. The remaining 8 ravens (5 males, 3 females) were bred in captivity (Table 1) and released in October according to the procedure employed to reintroduce ravens (Koch et al. 1986). The released individuals originated from three breeding pairs in Germany. They were raised by their parents and spent their first summer in captivity with their family. In September, they were transported to the Konrad Lorenz Research Station, where they were kept together in a spacious aviary (80 m2) for one month before they were each released (one at a time) into free flight. All birds integrated into the non-breeding groups within one month, i.e. they joined the wild ravens for foraging, socialising and roosting and no longer returned to the aviary. Releasing juvenile ravens during their first autumn allowed us to create a situation with totally inexperienced ravens. Without following wild juveniles continuously from fledging, it is difficult to exactly determine their level of experience; but at least they spent some time in this area, which is why we classified them as experienced juvenile non-breeders.
We located the radio-tagged ravens from the bottom of the valley and investigated within an area of around 3000 ha (detection range for radio signals, limited by mountains) (Fig. 1), but did not track individuals outside of this area. The time between September 2011 and January 2014 was divided into seasons of 3 months, during which we tried to track all tagged individuals at least 36 times per season, equally spread across mornings after the main feeding event, around noon and in the evenings before sunset. We recorded a maximum of 3 locations per individual and day, which had to be at least 2 h apart. A handheld portable three-element Yagi antenna and a radio-tracking receiver (Sika, Biotrack Ltd) were used to locate the birds and, ideally, get close enough to visually identify them. The order in which the birds were located was randomised. When a direct observation was not possible, we used triangulation (walking or driving to at least two different positions within a few minutes) to estimate the position of the bird. We subsequently divided our recordings into four accuracy categories: A = direct observation; B = relocation estimated ± 25 m; C = relocation estimated ± 50 m; D = relocation estimated ± 100 m. Only 5.3 % of the data had the lowest accuracy (D), and these mostly concerned locations at a night roost, where ravens are very sensitive to disturbances. Category A represented 37.7 %, B 26.7 % and C 30.3 % of the data, thus providing sufficient accuracy for the analysis of our research questions. When a signal could not be detected, we proceeded with the next individual and tried again after the last individual had been located. The relocations were registered on a detailed map (Map Data© 2011, Google, scale 1:10,000) and then transferred into ArcGIS 10.1 (ESRI 2012) to extract coordinates.
In comparison with the behaviour of untagged ravens at the daily observations, we have no indication that the radio tags impeded the ravens’ behaviour in any way (personal observation). However, in three cases we noticed injuries during recapture and therefore removed the tag: one female (Qu) had an infected wound on her back; two females (Fk, Ti) had abrasions on the sternum. All three ravens recovered fully and became subjects of other studies in the following months and years.
Data analysis
Activity-range estimation
In this study we were interested in a certain area within a specific time frame, namely the “stop” phase during dispersal, which is why we chose the term “local activity range” rather than “home range”. Whereas an animal’s home range is often defined as the area used by an animal during normal activities such as food gathering, mating and caring for its young (Burt 1943; Fieberg and Börger 2012), the last two components were not included here, and we only considered relocations in a predefined area.
A method that is commonly employed to measure an animal’s space use is the utilisation distribution (UD), which represents the probability of finding an animal at a particular location at any randomly chosen time (Worton 1989; Powell and Mitchell 2012). Using this approach, we calculated the average size of the non-breeder’s activity range and core area in this stop phase. Using the ks package (Duong 2007) in the statistics programme R (R Development Core Team 2014), we estimated the utilisation distribution (UD) of each individual via fixed kernel-density estimation (Silverman 1986; Worton 1989) and the plug-in method to select the smoothing parameters (Wand and Jones 1995; Gitzen et al. 2006). The plug-in method has been shown to be more reliable than more traditionally used methods (“first-generation” methods such as least-squares cross-validation) (Jones et al. 1996), and even more so when its unconstrained version is used (Duong 2007). Local activity range and its core area were defined as the areas within the 95 and 50 % contours of the estimated UD, respectively. We used n > 15 relocations as a minimum sample size for kernel estimation. To our knowledge there is no recommended minimum sample size for the plug-in bandwidth selector, although a minimum size of n > 30 is recommended for the least-squares cross-validation bandwidth selector (Seaman et al. 1999). Also, in a study of ravens that utilised another bandwidth selector (HREF), a minimum of n = 10 relocations was shown to be sufficient (Webb et al. 2011). Following Garton et al. (2001) and Webb et al. (2011), we found no correlation between sample size and local activity range (R 2 = 0.30, p = 0.193, Spearman’s rank correlation) or core area (R 2 = −0.14, p = 0.550).
Spatial overlap among individuals
Several different statistics have been proposed to quantify the overlap of home ranges (reviewed in Fieberg and Kochanny 2005). As it is a simple and intuitive method, we initially used a two-dimensional overlap framework, which entailed calculating the proportion of one animal’s local activity range that overlapped with another animal’s local activity range. The results of this method are easy to interpret but can be misleading, especially when minimum convex polygons are used, since large overlap estimates can occur despite a low probability of finding two individuals in the same area if UDs are not taken into account (Kernohan et al. 2001). Therefore, we also used the three-dimensional overlap, which contains more detailed information. Utilising these methods, paired UDs can be ranked in terms of their degree of overlap (Kernohan et al. 2001). Using simulations, Fieberg and Kochanny (2005) compared several three-dimensional overlap indices and found them to be biased too low when there was a high degree of overlap and too high when there was a low degree of overlap. Additionally, these effects strongly depend on sample size, making comparisons across studies with different sample sizes problematic (Fieberg and Kochanny 2005). To avoid these drawbacks, we applied another approach that involved asking a slightly different question. Instead of how much individuals overlapped, we wanted to know whether their utilisation distributions were equal (null hypothesis) or different (alternative hypothesis). Using the advantages of kernel smoothing, this approach has recently been realised in the context of quantitative cell comparisons (Duong et al. 2012), but, to our knowledge, it has not been implemented in an ecological study. The method (the kernel density based global two-sample comparison test) is designed to handle unbalanced sample sizes, although its overall accuracy is limited by the smallest sample size (Duong personal communication). The kernels of each individual are compared in a multivariate, nonparametric two-sample test. In other words, the test statistic calculates the probability that two UDs (three-dimensional overlap) are from the same distribution (Duong et al. 2012).
Additionally, we used this approach to compare the 95 % UDs obtained for each individual in different seasons, i.e. summer (April–September) versus winter (October–March), when the number of relocations allowed a reasonable comparison.
Modelling approach
We tested which of the factors had an impact on the size of the local activity range (n = 21) by creating generalised linear models (family = gamma, link = logit) with the following fixed factors: age (juvenile versus sub/adults), sex, origin (“wild” representing experienced versus “captive-bred” representing unexperienced), number of relocations, percentage of the search trials in which the individual was present (as a proxy for the strength of site fidelity) and the number of days between the first and last relocation of the individual. Due to the large number of fixed factors compared to the sample size, we calculated the events per variable ratio (EPV) as the number of outcome events per parameter estimated for fixed effects (Vittinghoff and McCulloch 2007). We aimed for an EPV of at least 5 (Vittinghoff and McCulloch 2007), which meant that the maximum number of fixed factors per model was set to 4.
To assess which of the factors may influence whether the UDs of the local activity ranges of two individuals are equal, we used the calculated p values from the comparisons of each individual with all other individuals (n = 210) as a dependent variable in generalised linear mixed models (family = beta, link = logit). Thus, we tested which of the factors influence the spatial overlap between individuals using two random factors (the identities of the two individuals being compared) and four fixed factors (age, sex, origin and number of relocations, including possible interactions).
We used an information-theoretic approach to evaluate alternative models based on the Kullback–Leibler relative distance (Burnham and Anderson 2002), as well as the second-order form of Akaike’s information criterion (AICc; Hurvich and Tsai 1989) to account for small sample sizes when estimating model parameters. We ranked all models according to their AICc values and selected the models with ΔAICc <2 with respect to the top-ranked model for model averaging in order to create model-averaged coefficients (Burnham and Anderson 2002). All analyses were performed in R (version 3.1.0; R Development Core Team 2014), and the scripts are published in the Electronic supplementary material (ESM).
Results
Activity-range estimation and distribution in the study area
In total we obtained 2184 relocations of 21 individuals by radio-tracking between September 2011 and January 2014. On average, the ravens were present in the study area in 69.2 % of the relocation attempts (range per individual: 27.2–98.7 %, Table 1).
The local activity range (95 % UD) was 27.0 ha on average (range 6.7–59.7 ha), with a mean core area (50 % UD) of 3.6 ha (range 1.2–15.7 ha). All individuals combined used an area of 48.0.9 ha (95 % UD), with a core area (50 % UD) of 2.6 ha, representing only a small fraction of the whole study area (3000 ha). As we expected, all core areas were concentrated around the main food sources (wild boar and wolf enclosure in the game park, see Fig. 2). Note that data were collected throughout the whole day but not during the main feeding events.
Activity-range estimates are shown for 95 % UD (a) and for 50 % UD (b), with lines of different colours for different individuals. Black areas mark the main food sources (enclosures of wild boars, bears and wolves), while the grey area reflects the detection range for radio signals (limited by mountains) (colour figure online)
Do individuals’ local activity ranges overlap?
Intersecting the two-dimensional local activity ranges (95 % UD) of all individuals with each other produced an average overlap of 61.1 ± 24.6 % SD (standard deviation), and 54.7 ± 27.4 % SD for the core areas (50 % UD). None of the birds were fully spatially segregated. The three-dimensional comparison, where we tested whether the UDs of two individuals have the same distribution or similar distributions, revealed that 52.4 % of all possible combinations were significantly different (p < 0.05) from each other. In other words, even though there was substantial overlap in the areas used by the birds (local activity range), more than half of the compared UDs (three-dimensional overlap) can be clearly distinguished from each other, indicating individual differences/preferences in space use at a fine scale.
Eight individuals each had a sufficient number of relocations that we were able to compare the local activity range between seasons, which revealed a significant difference between the space use in summer and winter for four individuals: Gl: p = 0.033, Ht: p = 0.014, Mo and Sh: p < 0.001; p > 0.05 for the other four subjects.
Which factors influence the local activity ranges and the overlap between them?
The model that best explained the size of the local activity range had only the origin (wild versus captive birds) as a fixed factor and was averaged with the second-best model (AIC weight = 0.12), which included the origin and % presence. The estimated size of the local activity range of wild ravens is 42.2 ha (confidence interval: 25.2–70.8 ha), which is significantly larger (p = 0.019) than that of released individuals, 23.0 ha (CI 9.3–57.3 ha, Table 2).
The similarity between two UDs was best explained by a model that included the fixed factors origin, age, number of relocations and an interaction of the latter two (Table 3). The UDs of two juveniles were more similar than those of two sub/adults. When comparing a juvenile’s UD with a sub/adult’s UD, the difference was even greater when age class interacted with the number of relocations (Table 3). The number of relocations itself also had a decreasing effect on the similarity of two UDs. Taken together, these results indicate that the UDs of juveniles were more similar to each other than sub/adult UDs were, but juvenile UDs clearly differed from sub/adult UDs. A high number of relocations might be important when trying to clearly define UDs.
Discussion
Most of our radio-tagged ravens could be reliably tracked at our study site over the course of several months or even years. The size of the local activity range of a raven was, in general, relatively small and concentrated around the main food sources throughout the day. When the locations of the UDs were taken into account, age class and experience (wild versus captive-bred) were found to affect space use, i.e. older birds seemingly preferred particular locations within the commonly used area.
Our findings stand in stark contrast to previous studies on non-breeding ravens’ space use. Compared to the small mean local activity range of 27 ha observed at our site, other studies report very large home ranges, ranging from 120 to 125,200 ha (see Boarman and Heinrich 1999 and references therein). Even though the local activity ranges used here were not originally intended to illustrate the entire home range of the tagged non-breeders, these individuals were present in the study area in around 70 % of the cases when we tried to locate them, showing very high site fidelity. Thus, our data describe the most commonly used part of these birds’ home ranges; in some cases, this is almost the entire home range during the period of data collection. This suggests that, under favourable conditions such as those present in our study area, with a rich food source and availability of suitable night roosts, non-breeding ravens may settle in very small areas for long time periods.
Previous studies have shown that the huge variation in reported home range size can be explained by ecological factors (e.g. habitat suitability, food abundance, nesting opportunity), data quality and analysis (e.g. number of observations) and social factors such as breeding status (Roth et al. 2004; Webb et al. 2012). In a primeval temperate forest in Eastern Europe, the home range size of breeding ravens was on average around 1300 ha (Rösner and Selva 2005), while in Western Marin County, CA, USA, it ranged from 15 to 480 ha (Roth et al. 2004). In some arctic areas such as Greenland, ravens even migrate seasonally (Restani et al. 2001). Non-breeders usually have larger home ranges than breeders, due to the long distances travelled (up to hundreds of kilometres) in the search for food and/or breeding opportunities (Heinrich et al. 1994; Webb et al. 2012). Our data show that this does not seem to be a general pattern, and that non-breeders can flexibly adopt different lifestyles. However, large individual differences were even found to occur under the favourable conditions present in our study area, and some ravens remain highly vagrant “globetrotters” (Braun et al. 2012).
The fact that non-breeders spent most of the time in a small area when they were at our study site can be explained by a “stop” phase during the dispersal process, most likely caused by the presence of a rich food source, which may increase the chance that a raven will find a social partner. During such a stop phase, movement patterns seem to be more similar to those of settled breeders with defined home ranges than to those of vagrant non-breeders, which has also been shown for other species (Smith 1978; Arcese 1989; Delgado et al. 2009). In particular, the small size of the core areas—which were all concentrated around the main food source—suggests that ravens may easily learn about the local environment and thereby probably improve their foraging efficiency. Indeed, the costs of dispersal when travelling through unknown areas (e.g. Pärt 1995; Stamps et al. 2005) can be reduced by becoming familiar with an area (Delgado et al. 2009). However, it is likely that there is a trade-off between familiarity with an area of high foraging potential and a high number of competitors for food and territories as well as the costs of possible agonistic interactions.
Ravens released from captivity had a significantly smaller local activity range than wild ravens, which could suggest that individuals that are unfamiliar with an area initially focus on the most important sites to increase their experience (i.e. the main food source and the night roost) instead of exploring other areas. Alternatively, this could be an artefact of the release process, because these birds were used to being restricted in the distance they could fly and to being fed in one place. As predicted, neither the sex of a bird nor its age class had any effect on the size of the local activity range. These findings indicate that stop phases with limited space use can occur throughout the dispersal process and over several years of life.
We found that individuals’ two-dimensional local activity ranges overlapped considerably (Fig. 2), and that a substantial part of the area was shared by all individuals. The first part of the result fits with what is already known for non-breeding ravens (e.g. Heinrich 1989; Wright et al. 2003), which usually form groups at food sources and roosts. However, our finding that even though all radio-tagged non-breeders could be mostly found in the same area, many of the birds displayed differing preferences for particular locations within this area (meaning that UDs differed among individuals) is completely new. Our averaged model revealed that sub/adults’ UDs differed from those of juveniles. Whereas sub/adult non-breeders could be found at individually distinct locations, juveniles tended to share the same space. The relocations of juvenile ravens occurred during their first fall and winter, a critical period for foraging after becoming independent from their parents. Enhanced social learning between siblings during this period has been observed for birds in captivity (Schwab et al. 2008). Since juveniles are the lowest in rank (Braun and Bugnyar 2012), they might also avoid older and higher-ranking individuals to prevent agonistic encounters, and therefore stay in groups with individuals of the same age. In contrast to juveniles, subadult ravens showed individual preferences for particular locations within their (already small) home range. While high aggression rates can be observed during foraging, socio-positive interactions occur primarily during the rest of the day (Braun et al. 2012; Braun and Bugnyar 2012), suggesting that ravens might choose their preferred sites according to social bonds and/or competitor presence. However, currently we cannot exclude other reasons, such as specific habitat features or staying close to their food caches, as underlying factors.
In half of the birds, we found that the utilisation distribution differed between winter and summer, which may be due to the presence of additional ephemeral food sources in the study area in winter. Especially during the main hunting season from September to January, ravens regularly feed on leftovers provided by hunters. Scavenging on such food sources seems to be widespread in ravens (Heinrich 1989), and can even lead to the association of gunshots with food (White 2005).
While non-breeding ravens have mostly been described as highly vagrant in the literature, we focused on birds at a permanent food source, which may resemble a stop phase in dispersal. During this phase, raven non-breeders showed similarly restricted space use to that seen for breeders. Thus, non-breeding ravens appear to flexibly choose between wandering around and settling temporarily in certain areas. This behavioural flexibility may be important for their survival in highly variable habitats and climate conditions. Furthermore, the (temporarily) restricted space use of non-breeders supports the notion that these birds may encounter each other regularly, which would set the stage for the development of socio-cognitive strategies during competition and cooperation.
References
Alberts SC, Altmann J (1995) Balancing costs and opportunities: dispersal in male baboons. Am Nat 145:279–306
Arcese P (1987) Age, intrusion pressure and defence against floaters by territorial male song sparrows. Anim Behav 35:773–784
Arcese P (1989) Territory acquisition and loss in male song sparrows. Anim Behav 37:56–63
Baltensperger AP, Mullet TC, Schmid MS et al (2013) Seasonal observations and machine-learning-based spatial model predictions for the common raven (Corvus corax) in the urban, sub-arctic environment of Fairbanks, Alaska. Polar Biol 36:1587–1599. doi:10.1007/s00300-013-1376-7
Bijlsma RG, Seldam H (2013) Impact of focal food bonanzas on breeding Ravens Corvus corax. Ardea 101:55–59
Boarman WI, Heinrich B (1999) Common Raven (Corvus corax). Birds North Am 476:1–32
Boarman WI, Patten MA, Camp RJ, Collis SJ (2006) Ecology of a population of subsidized predators: common ravens in the central Mojave Desert, California. J Arid Environ 67:248–261. doi:10.1016/j.jaridenv.2006.09.024
Bowler DE, Benton TG (2005) Causes and consequences of animal dispersal strategies: relating individual behaviour to spatial dynamics. Biol Rev Camb Philos Soc 80:205–225
Braun A, Bugnyar T (2012) Social bonds and rank acquisition in raven nonbreeder aggregations. Anim Behav 84:1507–1515. doi:10.1016/j.anbehav.2012.09.024
Braun A, Walsdorff T, Fraser ON, Bugnyar T (2012) Socialized sub-groups in a temporary stable raven flock? J Ornithol 153:97–104. doi:10.1007/s10336-011-0810-2
Buehler DA, Fraser JD, Fuller MR et al (1995) Captive and field-tested radio transmitter attachments for bald eagles. J F Ornithol 66:173–180
Burnham KP, Anderson DR (2002) Model selection and multimodel inference: a practical information-theoretic approach. Springer, New York
Burt WH (1943) Territoriality and home range concepts as applied to mammals. J Mammal 24:346–352
Caffrey C (2000) Marking crows. North Am Bird Bander 26:146–148
Campioni L, Delgado MDM, Penteriani V (2010) Social status influences microhabitat selection: breeder and floater Eagle Owls Bubo bubo use different post sites. Ibis (Lond 1859) 152:569–579. doi:10.1111/j.1474-919X.2010.01030.x
Campioni L, Lourenço R, Delgado MDM, Penteriani V (2012) Breeders and floaters use different habitat cover: should habitat use be a social status-dependent strategy? J Ornithol 153:1215–1223. doi:10.1007/s10336-012-0852-0
Clobert J, Le Galliard J-F, Cote J et al (2009) Informed dispersal, heterogeneity in animal dispersal syndromes and the dynamics of spatially structured populations. Ecol Lett 12:197–209. doi:10.1111/j.1461-0248.2008.01267.x
Cornell HN, Marzluff JM, Pecoraro S (2012) Social learning spreads knowledge about dangerous humans among American crows. Proc Biol Sci/R Soc 279(1728):499–508
Dall SRX, Wright J (2009) Rich pickings near large communal roosts favor “gang” foraging by juvenile common ravens. Corvus corax. PLoS One 4:e4530. doi:10.1371/journal.pone.0004530
Delgado M del M, Penteriani V (2008) Behavioral states help translate dispersal movements into spatial distribution patterns of floaters. Am Nat 172:475–485. doi:10.1086/590964
Delgado MDM, Penteriani V, Nams VO, Campioni L (2009) Changes of movement patterns from early dispersal to settlement. Behav Ecol Sociobiol 64:35–43. doi:10.1007/s00265-009-0815-5
Drack G, Kotrschal K (1995) Aktivitätsmuster und Spiel freilebender Kolkraben Corvus corax im inneren Almtal/Oberösterreich. Monticula 7:159–174
Duong T (2007) ks: kernel density estimation and kernel discriminant analysis for multivariate data in R. J Stat Softw 21:1–16
Duong T, Goud B, Schauer K (2012) Closed-form density-based framework for automatic detection of cellular morphology changes. Proc Natl Acad Sci USA 109:8382–8387. doi:10.1073/pnas.1117796109
Fieberg J, Börger L (2012) Could you please phrase “home range” as a question? J Mammal 93:890–902. doi:10.1644/11-MAMM-S-172.1
Fieberg J, Kochanny CO (2005) Quantifying home-range overlap: the importance of the utilization distribution. J Wildl Manage 69:1346–1359
Garton EO, Wisdom MJ, Leban FA, Johnson BK (2001) Experimental design for radiotelemetry studies. In: Millspaugh JJ, Marzluff JM (eds) Radio-tracking and animal populations. Academic, London, pp 15–42
Gitzen RA, Millspaugh JJ, Kernohan BJ (2006) Bandwidth selection for fixed-kernel analysis of animal utilization distributions. J Wildl Manage 70:1334–1344
Griffiths R, Double MC, Orr K, Dawson RJG (1998) A DNA test to sex most birds. Mol Ecol 7:1071–1075
Haffer J, Kirchner H (1993) Corvus corax—Kolkrabe. In: Glutz von Blotzheim UN, Bauer KM (eds) Handb. der Vögel Mitteleuropas. AULA-Verlag, Wiesbaden, pp 1947–2022
Heinrich B (1988) Winter foraging at carcasses by three sympatric corvids, with emphasis on recruitment by the raven, Corvus corax. Behav Ecol Sociobiol 23:141–156
Heinrich B (1989) Ravens in winter. Summit Books (Simon and Schuster), New York
Heinrich B (1994) When is the common raven black? Wilson Bull 106:571–572
Heinrich B, Marzluff JM (1992) Age and mouth color in common ravens. Condor 94:549–550
Heinrich B, Kaye D, Knight T, Schaumburg K (1994) Dispersal and association among common ravens. Condor 96:545–551
Huber B (1991) Bildung, Alterszusammensetzung und Sozialstruktur von Gruppen nichtbrütender Kolkraben. Metelener Schriftenr für Naturschutz 2:45–59
Hurvich CM, Tsai C-L (1989) Regression and time series model selection in small samples. Biometrika 76:297–307. doi:10.1093/biomet/76.2.297
Jones MC, Marron JS, Sheather SJ (1996) A brief survey of bandwidth selection for density estimation. J Am Stat Assoc 91:401–407. doi:10.2307/2291420
Kenward RE (2001) A manual for wildlife radio tagging. Academic, London
Kernohan BJ, Gitzen RA, Millspaugh JJ (2001) Analysis of animal space use and movements. In: Millspaugh JJ, Marzluff JM (eds) Radio-tracking and animal populations. Academic, London, pp 125–166
Koch A, Schuster A, Glandt D (1986) The state of the raven (Corvus corax L.) in central Europe with specific reference to a reintroduction measure in North Rhine-Westphalia. Z Jagdwiss 32:215–228
Marzluff JM, Neatherlin E (2006) Corvid response to human settlements and campgrounds: causes, consequences, and challenges for conservation. Biol Conserv 130:301–314
Marzluff JM, Heinrich B, Marzluff CS (1996) Raven roosts are mobile information centres. Anim Behav 51:89–103
Marzluff JM, Walls J, Cornell HN, Withey JC, Craig DP (2010) Lasting recognition of threatening people by wild American crows. Anim Behav 79(3):699–707. doi:10.1016/j.anbehav.2009.12.022
Pärt T (1995) The importance of local familiarity and search costs for age- and sex-biased philopatry in the collared flycatcher. Anim Behav 49:1029–1038
Penteriani V, Delgado MDM (2011) There is a limbo under the moon: what social interactions tell us about the floaters’ underworld. Behav Ecol Sociobiol 66:317–327. doi:10.1007/s00265-011-1279-y
Powell RA, Mitchell MS (2012) What is a home range? J Mammal 93:948–958. doi:10.1644/11-MAMM-S-177.1
R Development Core Team (2014) R: a language and environment for statistical computing 3.1.0. http://www.r-project.org
Ratcliffe D (1997) The raven. A natural history in Britain and Ireland. Poyser, London
Restani M, Marzluff JM, Yates RE (2001) Effects of anthropogenic food sources on movements, survivorship, and sociality of Common Ravens in the Arctic. Condor 103:399–404
Rösner S, Selva N (2005) Use of the bait-marking method to estimate the territory size of scavenging birds: a case study on ravens Corvus corax. Wildl Biol 11:183–191. doi:10.2981/0909-6396(2005)11[183:UOTBMT]2.0.CO;2
Roth JE, Kelly JP, Sydeman WJ, Colwell MA (2004) Sex differences in space use of breeding Common Ravens in western Marin County, California. Condor 106:529–539
Saarenmaa H, Stone ND, Folse LJ et al (1988) An artificial intelligence modelling approach to simulating animal/habitat interactions. Ecol Modell 44:125–141
Scarpignato AL, George TL (2013) Space use by common ravens in marbled murrelet nesting habitat in northern California. J F Ornithol 84:147–159. doi:10.1111/jofo.12013
Schwab C, Bugnyar T, Schloegl C, Kotrschal K (2008) Enhanced social learning between siblings in common ravens, Corvus corax. Anim Behav 75:501–508. doi:10.1016/j.anbehav.2007.06.006
Seaman DE, Millspaugh JJ, Kernohan BJ et al (1999) Effects of sample size on kernel home range estimates. J Wildl Manage 63:739–747
Silverman BW (1986) Density estimation for statistics and data analysis. Chapman and Hall, London
Smith SM (1978) The “underworld” in a territorial sparrow: adaptive strategy for floaters. Am Nat 112:571–582
Stahler D, Heinrich B, Smith D (2002) Common ravens, Corvus corax, preferentially associate with grey wolves, Canis lupus, as a foraging strategy in winter. Anim Behav 64:283–290. doi:10.1006/anbe.2002.3047
Stamps JA, Krishnan VV, Reid ML (2005) Search costs and habitat selection by dispersers. Ecology 86:510–518
Stiehl RB (1978) Aspects of the ecology of the common raven in Harney Basin. Portland State University, Portland
Vittinghoff E, McCulloch CE (2007) Relaxing the rule of ten events per variable in logistic and Cox regression. Am J Epidemiol 165:710–718. doi:10.1093/aje/kwk052
Vuilleumier S, Perrin N (2006) Effects of cognitive abilities on metapopulation connectivity. Oikos 113:139–147
Wand MP, Jones MC (1995) Kernel smoothing. Chapman and Hall, London
Waser PM, Creel SR, Lucas JR (1994) Death and disappearance—estimating mortality risks associated with philopatry and dispersal. Behav Ecol 5:135–141
Webb WC, Boarman WI, Rotenberry JT (2004) Common raven juvenile survival in a human-augmented landscape. Condor 106:517–528. doi:10.1650/7443
Webb WC, Boarman WI, Rotenberry JT (2009) Movements of juvenile common ravens in an arid landscape. J Wildl Manage 73:72–81. doi:10.2193/2007-549
Webb WC, Marzluff JM, Hepinstall-Cymerman J (2011) Linking resource use with demography in a synanthropic population of Common Ravens. Biol Conserv 144:2264–2273. doi:10.1016/j.biocon.2011.06.001
Webb WC, Marzluff JM, Hepinstall-cymerman J (2012) Differences in space use by Common Ravens in relation to sex, breeding status, and kinship. Condor 114:584–594. doi:10.1525/cond.2012.110116
White C (2005) Hunters ring dinner bell for ravens: experimental evidence of a unique foraging strategy. Ecology 86:1057–1060. doi:10.1890/03-3185
Worton BJ (1989) Kernel methods for estimating the utilization distribution in home-range studies. Ecology 70:164–168
Wright J, Stone RE, Brown N (2003) Communal roosts as structured information centres in the raven, Corvus corax. J Anim Ecol 72:1003–1014. doi:10.1046/j.1365-2656.2003.00771.x
Acknowledgments
We are grateful to Gesche Westphal-Fitch for her assistance with editing the manuscript and to two anonymous reviewers for their helpful comments on earlier drafts of the manuscript. We acknowledge support from the Herzog von Cumberland Stiftung for cooperation and the “Verein der Förderer KLF”. The current study was funded by the FWF (Austrian Science Fund) programs Y366-B17 and W1234.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest. All applicable international, national, and/or institutional guidelines for the care and use of animals were followed. All procedures performed in studies involving animals were in accordance with the ethical standards of the Austrian and local government guidelines and the institutional guidelines of the Core Facility KLF for Behaviour and Cognition, University of Vienna.
Additional information
Communicated by T. Gottschalk.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Loretto, MC., Reimann, S., Schuster, R. et al. Shared space, individually used: spatial behaviour of non-breeding ravens (Corvus corax) close to a permanent anthropogenic food source. J Ornithol 157, 439–450 (2016). https://doi.org/10.1007/s10336-015-1289-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10336-015-1289-z