Abstract
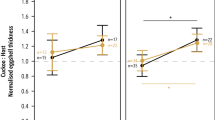
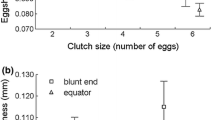
One of the most obvious adaptations to the brood parasitic mode of reproduction is the formation of eggs with unusually strong shells, which apparently reduce chances of egg breakage during laying and puncture ejection attempts of parasitic eggs by the hosts. We tested a hypothesis that strong eggshells of the Common Cuckoo, Cuculus canorus, may have also led to a stronger hatching muscle, musculus complexus. First, the Cuckoo hatching muscle had a higher density of fibers than that of the similarly sized Great Reed Warbler Acrocephalus arundinaceus chicks; and second, the cross-sectional area of fibers of the hatching muscle was smaller in the Cuckoo than in the Great Reed Warbler. We propose that the increased density of muscle fibers in the Cuckoo facilitates hatching out of structurally strong eggshells because chicks possessing this trait should be able to exert greater pressure on the shell during the hatching process. Our results are consistent with the hypothesis that the higher density of fibers in the musculus complexus represents another adaptation facilitating hatching from unusually strong parasitic eggs that has presumably evolved during coevolution involving the Cuckoo and its hosts.
Zusammenfassung
Wie ein Kuckuck ( Cuculus canorus ) aus dem Ei schlüpft: die Folgen einer harten Eierschale für den Schlupfmuskel ( Musculus complexus ) Eine der offensichtlichsten Anpassungen an eine brutparasitische Lebensweise ist die Bildung von Eiern mit einer ungewöhnlich harten Schale, was offenbar das Risiko verringert, dass die Eier während des Legens zerbrechen oder vom Wirt durch Aufspießen mit dem Schnabel aus dem Nest entfernt werden. Wir haben die Hypothese getestet, dass die harte Eischale des Kuckucks (Cuculus canorus) auch zu einem kräftigeren Schlupfmuskel (Musculus complexus) geführt haben könnte. Erstens wies der Schlupfmuskel des Kuckucks eine höhere Faserdichte auf als der ähnlich großer Küken des Drosselrohrsängers (Acrocephalus arundinaceus), und zweitens war die Querschnittsfläche der Fasern des Schlupfmuskels beim Kuckuck kleiner als beim Drosselrohrsänger. Wir schlagen vor, dass die höhere Muskelfaserdichte beim Kuckuck das Schlüpfen aus Eiern mit härterer Schale erleichtert, da Küken mit diesem Merkmal in der Lage sein sollten, währen des Schlupfprozesses höheren Druck auf die Schale auszuüben. Unsere Ergebnisse sind im Einklang mit der Hypothese, dass die höhere Faserdichte im Musculus complexus eine weitere Anpassung darstellt, die das Schlüpfen aus ungewöhnlich hartschaligen Brutparasiteneiern erleichtert und die vermutlich während der Koevolution von Kuckuck und Wirt entstanden ist.

Similar content being viewed by others
References
Anderson MG, Moskát C, Bán M, Grim T, Cassey P, Hauber ME (2009) Egg eviction imposes a recoverable cost of virulence in chicks of a brood parasite. PLoS ONE 4(11):e7725
Antonov A, Stokke BG, Moksnes A, Kleven O, Honza M, Røskaft E (2006) Eggshell strength of an obligate brood parasite: a test of the puncture resistance hypothesis. Behav Ecol Sociobiol 60:11–18
Ashmore CR, Adrie PB, Doerr L, Stokem H (1973) Development of muscle fibers in the complexus muscle of normal and dystrophic chicks. J Histochem Cytochem 21:266–278
Bancroft JD, Cook HC, Stirling RW, Turner DR (1994) Manual of histological techniques and their diagnostic application. Churchill Livingstone, Edinburgh
Birkhead TR, Hemmings N, Spottiswoode CN, Mikulica O, Moskát C, Bán M, Schulze-Hagen K (2011) Internal incubation and early hatching in brood parasitic birds. Proc R Soc Lond B 278:1019–1024
Blackburn DG, Darrell RS, Lonergan KT, Mancini RP, Sidor CA (1995) Differential testosterone sensitivity of forelimb muscles of male Leopard Frogs, Rana pipiens: test of a model system. Amphib–Reptil 16:351–356
Bock WJ (1974) The avian skeletomuscular system. In: Farner DS, King JR (eds) Avian biology, vol IV. Academic, Dublin, pp 120–250
Bock WJ, Hikida RS (1968) An analysis of twitch and tonus fibers in the hatching muscle. Condor 70:211–222
Bock WJ, Hikida RS (1969) Turgidity and function of the hatching muscle. Am Midl Natur 81:99–106
Brantley RK, Marchaterre MA, Bass AH (1993) Androgen effects on vocal muscle structure in a teleost fish with inter- and intra-sexual dimorphism. J Morphol 216:305–318
Brooker MG, Brooker LC (1991) Eggshell strength in cuckoos and cowbirds. Ibis 133:406–413
Connaughton MA, Taylor MH (1995) Effects of exogenous testosterone on sonic muscle mass in the Weakfish, Cygnoscion regalis. Gen Comp Endocrinol 100:238–245
Davies NB (2011) Cuckoo adaptations: trickery and tuning. J Zool 284:1–14
Fisher HI (1958) The hatching muscle in the chick. Auk 75:391–399
Fisher HI (1961) The hatching muscle in North American grebes. Condor 63:227–233
Fisher HI (1962) The hatching muscle in Franklin’s Gull. Wilson Bull 74:166–172
Fisher HI (1966) Hatching and the hatching muscle in some North American ducks. Trans Ill State Acad Sci 59:305–325
Gaston AJ (1976) Brood parasitism by the pied crested Cuckoo Clamator jacobinus. J Anim Ecol 45:331–348
George JC, Iype PT (1963) The mechanism of hatching in the chick. Pavo 1:52–56
Gosler AG, Higham JP, Reynolds SJ (2005) Why are bird’s eggs speckled? Ecol Lett 8:1105–1113
Gross GH (1985) Innervation of the complexus (“hatching”) muscle of the chick. J Comp Neurol 232:180–189
Hargitai R, Moskát C, Bán M, Gil D, López-Rull I, Solymos E (2010) Eggshell characteristics and yolk composition in the common cuckoo Cuculus canorus: are they adapted to brood parasitism? J Avian Biol 41:177–185
Hargitai R, Costantini D, Moskát C, Bán M, Muriel J, Hauber ME (2012) Variation in plasma oxidative status and testosterone level in relation to egg-eviction effort and age of brood-parasitic Common Cuckoo nestlings. Condor 114:782–791
Honza M, Picman J, Grim T, Novák V, Čapek M Jr, Mrlík V (2001) How to hatch from an egg of great structural strength. A study of the Common Cuckoo. J Avian Biol 32:249–255
Hsaio CYY, Ungar F (1969) Lipid changes in the chick hatching muscle. Proc Soc Exp Biol Med 132:1047–1051
Igic B, Braganza K, Hyland MM, Silyn-Roberts H, Cassey P, Grim T, Rutila J, Moskát C, Hauber ME (2011) Alternative mechanisms of increased eggshell hardness of avian brood parasites relative to host species. J R Soc Interface. doi:10.1098/rsif.2011.0207
John TM, George JC, Moran ET Jr (1987) Pre- and post-hatch ultrastructural and metabolic changes in the hatching muscle of turkey embryos from antibiotic and glucose treated eggs. Cytobios 49:197–210
Kawata M (1995) Roles of steroid hormones and their receptors in structural organization in the nervous system. Neurosci Res 24:1–46
Keibel F (1912) Wie zerbricht der ausschlüpfende Vogel die Eischale? Anat Anz 41:381–382
Krüger O, Davies NB (2004) The evolution of egg size in the brood parasitic cuckoos. Behav Ecol 15:210–218
Lipar JL (2001) Yolk steroids and the development of the hatching muscle in nestling European Starling. J Avian Biol 32:231–238
Lipar JL, Ketterson ED (2000) Maternally derived yolk testosterone enhances the development of the hatching muscle in the Red-winged Blackbird Agelaius phoeniceus. Proc R Soc Lond B 267:2005–2010
Makatsch W (1955) Der Brutparasitismus in der Vogelwelt. Neumann, Radebeul
Maurer G, Portugal SJ, Cassey P (2011) Review: an embryo’s eye view of avian eggshell pigmentation. J Avian Biol 42:494–504
Øien IJ, Moksnes A, Røskaft E, Honza M (1998) Costs of Cuckoo Cuculus canorus parasitism to Reed Warblers Acrocephalus scirpaceus. J Avian Biol 29:209–215
Payne RB (1974) The evolution of clutch size and reproductive rates in parasitic cuckoos. Evolution 28:169–181
Picman J (1997) Are cowbird eggs unusually strong from the inside? Auk 114:66–73
Picman J, Pribil S (1997) Is great eggshell density an alternative mechanism by which parasitic cuckoos increase the strength of their eggs? J Ornithol 138:531–541
Pinheiro J, Bates D, DebRoy S, Sarkar D, Team RDC (2010) Package nlme. Linear and nonlinear mixed effects models. Version 3.1-97
Pohlman AG (1919) Concerning the causal factor in the hatching of the chick with particular reference to the Musculus complexus. Anat Rec 17:89–104
Portugal SJ, Hauber ME, Maurer G, Stokke BG, Grim T, Cassey P (2014) Rapid development of brood-parasitic cuckoo embryos cannot be explained by increased gas exchange through the eggshell. J Zool 293:219–226
Ramachandran S, Klicka J, Ungar F (1969) Biochemical changes in the musculus complexus of chick (Gallus domesticus). Comp Biochem Physiol 30:631–640
R Core Team (2013) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna
Romeis B (1989) Mikroskopische Technik. Urban and Schwarzenberg, München
Rothstein SI (1990) A model system for coevolution: Avian brood parasitism. Annu Rev Ecol Syst 21:481–508
Sidor CA, Blackburn DG (1998) Effects of testosterone administration and castration on the forelimb musculature of male Leopard Frogs, Rana pipiens. J Exp Zool 280:28–37
Smail JR (1964) A possible role of the Musculus complexus in pipping the chicken egg. Am Midl Nat 72:499–506
Török J, Moskát C, Michl G, Péczely P (2004) Common cuckoos (Cuculus canorus) lay eggs with larger yolk but no more testosterone than their great reed warbler (Acrocephalus arundinaceus) hosts. Ethol Ecol Evol 16:271–277
Wyllie I (1981) The cuckoo. Batsford, London
Acknowledgments
The study was supported by the Czech Science Foundation (Grant No. P506/12/2404), the Grant Agency of the Academy of Sciences of the Czech Republic (Grant No. IAA6093203), and by institutional support (RVO: 68081766).
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by F. Bairlein.
Rights and permissions
About this article
Cite this article
Honza, M., Feikusová, K., Procházka, P. et al. How to hatch from the Common Cuckoo (Cuculus canorus) egg: implications of strong eggshells for the hatching muscle (musculus complexus). J Ornithol 156, 679–685 (2015). https://doi.org/10.1007/s10336-015-1163-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10336-015-1163-z