Abstract
Objective
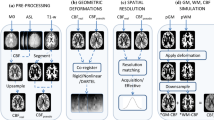
In this perfusion magnetic resonance imaging study, the performances of different pseudo-continuous arterial spin labeling (PCASL) sequences were compared: two-dimensional (2D) single-shot readout with simultaneous multislice (SMS), 2D single-shot echo-planar imaging (EPI) and multishot three-dimensional (3D) gradient and spin echo (GRASE) sequences combined with a background-suppression (BS) module.
Materials and methods
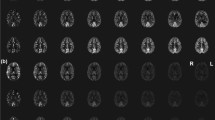
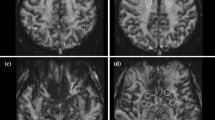
Whole-brain PCASL images were acquired from seven healthy volunteers. The performance of each protocol was evaluated by extracting regional cerebral blood flow (rCBF) measures using an inline morphometric segmentation prototype. Image data postprocessing and subsequent statistical analyses enabled comparisons at the regional and sub-regional levels.
Results
The main findings were as follows: (i) Mean global CBF obtained across methods was were highly correlated, and these correlations were significantly higher among the same readout sequences. (ii) Temporal signal-to-noise ratio and gray-matter-to-white-matter CBF ratio were found to be equivalent for all 2D variants but lower than those of 3D-GRASE.
Discussion
Our study demonstrates that the accelerated SMS readout can provide increased acquisition efficiency and/or a higher temporal resolution than conventional 2D and 3D readout sequences. Among all of the methods, 3D-GRASE showed the lowest variability in CBF measurements and thus highest robustness against noise.





Similar content being viewed by others
References
Salinet ASM, Panerai RB, Robinson TG (2014) The longitudinal evolution of cerebral blood flow regulation after acute ischaemic stroke. Cerebrovasc Dis Extra 4:186–197
Saa JP, Tse T, Baum C, Cumming T, Josman N, Rose M, Carey L (2019) Longitudinal evaluation of cognition after stroke—a systematic scoping review. PLoS ONE 14:e0221735
von Kummer R (2019) Treatment of ischemic stroke beyond 3 hours: is time really brain? Neuroradiology 61:115–117
Alsop DC, Detre JA, Golay X, Gunther M, Hendrikse J, Hernandez-Garcia L, Lu H, MacIntosh BJ, Parkes LM, Smits M, van Osch MJ, Wang DJ, Wong EC (2015) “Recommended implementation of arterial spin-labeled Perfusion mri for clinical applications: A consensus of the ISMRM Perfusion Study group and the European consortium for ASL in dementia. Magn Reson Med 73(1):102–116
Detre JA, Leigh JS, Williams DS, Koretsky AP (1992) Perfusion imaging. Magn Reson Med 23:37–45
Pollock JM, Tan H, Kraft R, Whitlow CT, Burdette JH, Maldjian JA (2010) Arterial spin labeled MRI Perfusion. Magn Reson Imaging Clin N Am. https://doi.org/10.1016/j.mric.2009.01.008.Arterial
Sadowski EA, Bennett LK, Chan MR, Wentland AL, Garrett AL, Garrett RW, Djamali A (2007) Nephrogenic systemic fibrosis: risk factors and incidence estimation. Radiology 243:148–157
Günther M, Oshio K, Feinberg DA (2005) Single-shot 3D imaging techniques improve arterial spin labeling perfusion measurements. Magn Reson Med 54:491–498
Fernández-Seara MA, Edlow BL, Hoang A, Wang J, Feinberg DA, Detre JA (2008) Minimizing acquisition time of arterial spin labeling at 3T. Magn Reson Med 59:1467–1471
Vidorreta M, Wang Z, Rodriguez I, Pastor MA, Detre JA, Fernández-Seara MA (2013) Comparison of 2D and 3D single-shot ASL perfusion fMRI sequences. Neuroimage 66:662–671
Dai W, Shankaranarayanan A, Alsop DC (2013) Volumetric measurement of perfusion and arterial transit delay using hadamard encoded continuous arterial spin labeling. Magn Reson Med 69:1014–1022
Wang J, Zhang Y, Wolf RL, Roc AC, Alsop DC, Detre JA (2005) Amplitude-modulated continuous arterial spin-labeling 3.0-T perfusion MR imaging with a single coil: feasibility study. Radiology 235:218–228
Dai W, Garcia DM, de Bazelaire C, Alsop DC (2008) Continuous flow driven inversion for arterial spin labelling using pulsed radiofrequency and gradient fields. Magn Reson Med 60:1488–1497
Garcia DM, Duhamel G, Alsop DC (2005) Efficiency of inversion pulses for background suppressed arterial spin labeling. Magn Reson Med 54:366–372
Maleki N, Dai W, Alsop DC (2012) Optimization of background suppression for arterial spin labeling perfusion imaging. Magn Reson Mater Phy 25:127–133
Ye FQ, Frank JA, Weinberger DR, McLaughlin AC (2000) Noise reduction in 3D perfusion imaging by attenuating the static signal in arterial spin tagging (ASSIST). Magn Reson Med 44:92–100
Kilroy E, Apostolova L, Liu C, Yan L, Ringman J, Wang D (2014) Reliability of 2D and 3D pseudo-continuous arterial spin labeling perfusion mri in elderly populations—comparison with 15O-water PET. J Magn Reson Imaging 39:931–939
Wu WC, Edlow BL, Elliot MA, Wang J, Detre JA (2009) Physiological modulations in arterial spin labeling perfusion magnetic resonance imaging. IEEE Trans Med Imaging 28:703–709
Young IR, Larkman DJ, Hajnal JV, Ehnholm G, Herlihy AH, Coutts GA (2002) Use of multicoil arrays for separation of signal from multiple slices simultaneously excited. J Magn Reson Imaging 13:313–317
Moeller S, Yacoub E, Olman CA, Auerbach E, Strupp J, Harel N, Uǧurbil K (2010) Multiband multislice GE-EPI at 7 tesla, with 16-fold acceleration using partial parallel imaging with application to high spatial and temporal whole-brain FMRI. Magn Reson Med 63:1144–1153
Setsompop K, Cohen-Adad J, Gagoski BA, Raij T, Yendiki A, Keil B, Wedeen VJ, Wald LL (2012) Improving diffusion MRI using simultaneous multi-slice echo planar imaging. Neuroimage 63:569–580
Kim T, Shin W, Zhao T, Beall EB, Lowe MJ, Bae KT (2013) Whole brain perfusion measurements using arterial spin labeling with multiband acquisition. Magn Reson Med 70:1653–1661
Feinberg DA, Beckett A, Chen L (2013) Arterial spin labeling with simultaneous multi-slice echo planar imaging. Magn Reson Med 70:1500–1506
Kim SG, Tsekos NV (1997) Perfusion imaging by a flow–sensitive alternating inversion recovery (FAIR) technique: application to functional brain imaging. Magn Reson Med 37:425–435
Li X, Wang D, Auerbach EJ, Moeller S, Ugurbil K, Metzger GJ (2015) Theoretical and experimental evaluation of multi-band EPI for high-resolution whole brain pCASL Imaging. Neuroimage 106:170–181
Lövblad KO, Montandon ML, Viallon M, Rodriguez C, Toma S, Golay X, Giannakopoulos P (2015) Arterial spin-labeling parameters influence signal variability and estimated regional relative cerebral blood flow in normal aging and mild cognitive impairment: fAIR versus PICORE techniques. AJNR Am J Neuroradiol 36(7):1231–1236
Schmitter D, Roche A, Maréchal B, Ribes D, Abdulkadir A, Bach-Cuadra M, Daducci A, Granziera C, Klöppel S, Maeder P, Meuli R, Krueger G (2015) An evaluation of volume-based morphometry for prediction of mild cognitive impairment and Alzheimer’s disease. NeuroImage Clin 7:7–17
Collins DLL, Holmes CJ, Peters TM (1995) Automatic 3-D model-based neuroanatomical segmentation. Hum Brain 3:190–208
Marques JP, Kober T, Krueger G, van der Zwaag W, Van de Moortele PF, Gruetter R (2010) MP2RAGE, a self bias-field corrected sequence for improved segmentation and T1-mapping at high field. Neuroimage 49:1271–1281
Hilbert T, Sumpf TJ, Weiland E, Frahm J, Thiran JP, Meuli R, Kober T, Krueger G (2018) Accelerated T 2 mapping combining parallel MRI and model-based reconstruction: GRAPPATINI. J Magn Reson Imaging 48:359–368
Chappell MA, Groves AR, MacIntosh BJ, Donahue MJ, Jezzard P, Woolrich MW (2011) Partial volume correction of multiple inversion time arterial spin labeling MRI data. Magn Reson Med 65:1173–1183
Lee IA, Preacher KJ (2013) Calculation for the test of the difference between two dependent correlations with one variable in common [computer soft-ware]. http://quantpsy.org
Dolui S, Vidorreta M, Wang Z, Nasrallah IM, Alavi A, Wolk DA, Detre JA (2017) Comparison of PASL, PCASL, and background-suppressed 3D PCASL in mild cognitive impairment. Hum Brain Mapp 38:5260–5273
Ferré JC, Bannier E, Raoult H, Mineur G, Carsin-Nicol B, Gauvrit JY (2013) Arterial spin labeling (ASL) perfusion: techniques and clinical use. Diagn Interv Imaging 94:1211–1223
Zhu J, Zhuo C, Qin W, Xu Y, Xu L, Liu X (2015) Altered resting-state cerebral blood flow and its connectivity in schizophrenia. J Psychiatr Res 63:28–35
Wolf Marc, Okazaki Shuhei, Eisele Philipp, Roßmanith Christina, Gregori Johannes, Griebe Martin, Günther Matthias, Gass Achim, Hennerici Michael, Szabo Kristina, Kern R (2018) Arterial spin labeling cerebral perfusion magnetic resonance imaging in migraine aura: an observational study. J Stroke Cerebrovasc Dis 27(10):101
Setsompop K, Gagoski BA, Polimeni JR, Witzel T, Wedeen VJ, Wald LL (2012) Blipped-controlled aliasing in parallel imaging for simultaneous multislice echo planar imaging with reduced g-factor penalty. Magn Reson Med 67:1210–1224
Dolui S, Wang Z, Wang DJJ, Mattay R, Finkel M, Elliott M, Desiderio L, Inglis B, Mueller B, Stafford RB, Launer LJ, Jacobs DR, Bryan RN, Detre JA (2016) Comparison of non-invasive MRI measurements of cerebral blood flow in a large multisite cohort. J Cereb Blood Flow Metab 36:1244–1256
Hales PW, Kirkham FJ, Clark CA (2016) A general model to calculate the spin-lattice (T1) relaxation time of blood, accounting for haematocrit, oxygen saturation and magnetic field strength. J Cereb Blood Flow Metab 36:370–374
Wu W-C, Jain V, Li C, Giannetta M, Hurt H, Wehrli FW, Wang DJJ (2010) In vivo venous blood T1 measurement using inversion recovery true-FISP in children and adults. Magn Reson Med 64:1140–1147
Jenkinson M, Bannister P, Brady M, Smith S (2002) Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage 17:825–841
Kieran KJ, Brunbergz JA (1997) Adult claustrophobia, anxiety and sedation in MRI. Magn Reson Imaging 15:51–54
Mutsaerts HJMM, Steketee RME, Heijtel DFR, Kuijer JPA, Van Osch MJP, Majoie CBLM, Smits M, Nederveen AJ (2014) Inter-vendor reproducibility of pseudo-continuous arterial spin labeling at 3 Tesla. PLoS One. https://doi.org/10.1371/journal.pone.0104108
Qiu D, Straka M, Zun Z, Bammer R, Moseley ME, Zaharchuk G (2012) CBF measurements using multidelay pseudocontinuous and velocity-selective arterial spin labeling in patients with long arterial transit delays: comparison with xenon CT CBF. J Magn Reson Imaging 36:110–119
Ivanov D, Poser BA, Huber L, Pfeuffer J, Uludağ K (2017) Optimization of simultaneous multislice EPI for concurrent functional perfusion and BOLD signal measurements at 7T. Magn Reson Med 78:121–129
Campbell AM, Beaulieu C (2006) Comparison of multislice and single-slice acquisitions for pulsed arterial spin labeling measurements of cerebral perfusion. Magn Reson Imaging 24:869–876
Acknowledgements
This work was performed within the framework of the LABEX PRIMES (ANR-11-LABX-0063) of Université de Lyon, within the program “Investissements d’Avenir” (ANR-11-IDEX-0007) operated by the French National Research Agency (ANR).
Funding
No funding was received for this study.
Author information
Authors and Affiliations
Contributions
MN: Study conception and design; acquisition of data; analysis and interpretation of data; drafting of manuscript. TT: Study conception and design; acquisition of data; drafting of manuscript; critical revision. JP: Critical revision, provided the prototype 2D and 3D ASL sequences and protocol parameter optimization. BM: Acquisition of data, provided the prototype MorphoBox for inline segmentation. TH: Acquisition of data, provided the prototype GRAPPATINI for fast T2 mapping. TK: Acquisition of data and MP2RAGE protocol parameter optimization; critical revision. FS: Analysis and interpretation of data; drafting of manuscript; critical revision. PC: Analysis and interpretation of data. MV: Study conception and design; acquisition of data; analysis and interpretation of data; drafting of manuscript; critical revision.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Nanjappa, M., Troalen, T., Pfeuffer, J. et al. Comparison of 2D simultaneous multi-slice and 3D GRASE readout schemes for pseudo-continuous arterial spin labeling of cerebral perfusion at 3 T. Magn Reson Mater Phy 34, 437–450 (2021). https://doi.org/10.1007/s10334-020-00888-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10334-020-00888-8