Abstract
Objective
To investigate whether a SPIO-labeling technique could enable MR visualization of the treatment margin after X-irradiation at a single dose of 30 Gy.
Materials and methods
Fifteen rats bearing N1-S1 hepatoma in either the left (group 1) or right (group 2) liver lobe were examined. Four hours after systemic SPIO administration, the left lobe was selectively irradiated at 30 Gy. Liver T2* maps were acquired 7 days later using a 9.4 T scanner. The livers were excised and examined histologically.
Results
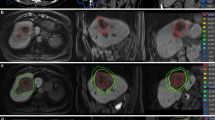
The irradiated area showed T2*-weighted hypointensity with significantly shorter T2* values than those in the non-irradiated area (p < 0.001). Tumors in group 1 completely disappeared, whereas tumors in group 2 had grown outside the T2*-weighted hypointensity by up to ~ 2.3 times that at baseline. Group 1 showed significantly higher probability of tumor regression than group 2 (p = 0.048). Histologically, iron deposition was heavier in irradiated areas than in non-irradiated areas.
Discussion
Even at a single dose of 30 Gy, which is a slightly higher dose than can be used clinically in stereotactic body radiotherapy, MR visualization of the treatment margin was achieved, because tumors showed significant growth outside the T2*-hypointense areas. In contrast, tumors disappeared inside the T2*-hypointense areas.






Similar content being viewed by others
References
Sanuki N, Takeda A, Kunieda E (2014) Role of stereotactic body radiation therapy for hepatocellular carcinoma. World J Gastroenterol 20(12):3100–3111
Takeda A, Sanuki N, Eriguchi T, Kobayashi T, Iwabutchi S, Matsunaga K, Mizuno T, Yashiro K, Nisimura S, Kunieda E (2014) Stereotactic ablative body radiotherapy for previously untreated solitary hepatocellular carcinoma. J Gastroenterol Hepatol 29(2):372–379
Scorsetti M, Comito T, Cozzi L, Clerici E, Tozzi A, Franzese C, Navarria P, Fogliata A, Tomatis S, D'Agostino G, Iftode C, Mancosu P, Ceriani R, Torzilli G (2015) The challenge of inoperable hepatocellular carcinoma (HCC): results of a single-institutional experience on stereotactic body radiation therapy (SBRT). J Cancer Res Clin Oncol 141(7):1301–1309
Takeda A, Sanuki N, Tsurugai Y, Iwabuchi S, Matsunaga K, Ebinuma H, Imajo K, Aoki Y, Saito H, Kunieda E (2016) Phase 2 study of stereotactic body radiotherapy and optional transarterial chemoembolization for solitary hepatocellular carcinoma not amenable to resection and radiofrequency ablation. Cancer 122(13):2041–2049
Herfarth KK, Debus J, Lohr F, Bahner ML, Rhein B, Fritz P, Hoss A, Schlegel W, Wannenmacher MF (2001) Stereotactic single-dose radiation therapy of liver tumors: results of a phase I/II trial. J Clin Oncol Off J Am Soc Clin Oncol 19(1):164–170
Furuta T, Yamaguchi M, Minami M, Ohtomo K, Fujii H (2017) Persistent T2*-hypointensity of the liver parenchyma after irradiation to the SPIO-accumulated liver: an imaging marker for responses to radiotherapy in hepatic malignancies. J Magn Reson Imaging JMRI 45(1):303–312
Mitsuda M, Yamaguchi M, Nakagami R, Furuta T, Sekine N, Niitsu M, Moriyama N, Fujii H (2013) Intensity correction method customized for multi-animal abdominal MR imaging with 3 T clinical scanner and multi-array coil. Magn Reson Med Sci 12(2):95–103
Lee H, Ahn YC, Oh D, Nam H, Kim YI, Park SY (2014) Tumor volume reduction rate measured during adaptive definitive radiation therapy as a potential prognosticator of locoregional control in patients with oropharyngeal cancer. Head Neck 36(4):499–504
Price P, McMillan TJ (1990) Use of the tetrazolium assay in measuring the response of human tumor cells to ionizing radiation. Cancer Res 50(5):1392–1396
Babsky AM, Ju S, Bennett S, George B, McLennan G, Bansal N (2012) Effect of implantation site and growth of hepatocellular carcinoma on apparent diffusion coefficient of water and sodium MRI. NMR Biomed 25(2):312–321
Babsky AM, Ju S, George B, Bennett S, Huang M, Jayaram HN, McLennan G, Bansal N (2011) Predicting response to benzamide riboside chemotherapy in hepatocellular carcinoma using apparent diffusion coefficient of water. Anticancer Res 31(6):2045–2051
Guo Y, Jin N, Klein R, Nicolai J, Yang GY, Omary RA, Larson AC (2012) Gas challenge-blood oxygen level-dependent (GC-BOLD) MRI in the rat Novikoff hepatoma model. Magn Reson Imaging 30(1):133–138
Guo Y, Zhang Y, Klein R, Nijm GM, Sahakian AV, Omary RA, Yang GY, Larson AC (2010) Irreversible electroporation therapy in the liver: longitudinal efficacy studies in a rat model of hepatocellular carcinoma. Cancer Res 70(4):1555–1563
Sheu AY, Zhang Z, Omary RA, Larson AC (2013) Invasive catheterization of the hepatic artery for preclinical investigation of liver-directed therapies in rodent models of liver cancer. Am J Transl Res 5(3):269–278
Clement O, Muhler A, Vexler VS, Rosenau W, Berthezene Y, Kuwatsuru R, Brasch RC (1992) Evaluation of radiation-induced liver injury with MR imaging: comparison of hepatocellular and reticuloendothelial contrast agents. Radiology 185(1):163–168
Stiskal M, Schwickert HC, Demsar F, Roberts TP, Szolar D, Weissleder R, Brasch RC (1996) Contrast enhancement in experimental radiation-induced liver injury: comparison of hepatocellular and reticuloendothelial particulate contrast agents. J Magn Reson Imaging 6(2):286–290
Stiskal M, Demsar F, Muhler A, Schwickert HC, Roberts TP, Szolar D, Fischer H, Brasch RC (1999) Contrast-enhanced MR imaging of two superparamagnetic RES-contrast agents: functional assessment of experimental radiation-induced liver injury. J Magn Reson Imaging 10(1):52–56
Yamaguchi M, Ohnuki K, Hotta K, Fujii H (2019) MR signal changes in superparamagnetic iron oxide nanoparticle-labeled macrophages in response to X irradiation. NMR Biomed 32(9):e4132
Masui T, Yan H, Kosugi I, Sakamoto S, Nishimura T, Takahashi M, Kaneko M, Fritz-Zieroth B (1996) Assessment of early radiation effects on the liver. Comparison of SPECT and MR. Acta Radiol 37(5):665–671
Padhani AR, Husband JE, Gueret Wardle D (1998) Radiation induced liver injury detected by particulate reticuloendothelial contrast agent. Br J Radiol 71(850):1089–1092
Jao JC, Lu HY, Lu HC, Liu GC, Chen SH, Lian SL, Yang SF, Chen PC (2010) Investigation of early liver radiation injury using resovist-enhanced MRI at 3T. In: 2010 4th International conference on bioinformatics and biomedical engineering, Chengdu, China, pp 1–4
Morimoto N, Ebara M, Kato H, Obata T, Fujita J, Kondo F, Tsujii H, Saisho H (1999) Early detection of radiation-induced liver injury in rat by superparamagnetic iron oxide-enhanced MR imaging. J Magn Reson Imaging 9(4):573–578
Magnitsky S, Walton RM, Wolfe JH, Poptani H (2007) Magnetic resonance imaging as a tool for monitoring stem cell migration. Neurodegener Dis 4(4):314–321
Hebard DW, Jackson KL, Christensen GM (1980) The chronological development of late radiation injury in the liver of the rat. Radiat Res 81(3):441–454
Buijs M, Geschwind JF, Syed LH, Ganapathy-Kanniappan S, Kunjithapatham R, Wijlemans JW, Kook Kwak B, Ota S, Vali M (2012) Spontaneous tumor regression in a syngeneic rat model of liver cancer: implications for survival studies. J Vasc Interv Radiol JVIR 23(12):1685–1691
Kelley JP, Trout ED (1972) Broad-beam attenuation in lead for x rays from 50 to 300 kVp. Radiology 104(1):171–175
Pepe A, Lombardi M, Positano V, Cracolici E, Capra M, Malizia R, Prossomariti L, De Marchi D, Midiri M, Maggio A (2006) Evaluation of the efficacy of oral deferiprone in beta-thalassemia major by multislice multiecho T2*. Eur J Haematol 76(3):183–192
Cheng AL, Batool S, McCreary CR, Lauzon ML, Frayne R, Goyal M, Smith EE (2013) Susceptibility-weighted imaging is more reliable than T2*-weighted gradient-recalled echo MRI for detecting microbleeds. Stroke 44(10):2782–2786
Acknowledgements
This study was supported by a Grant from the Japan Radiological Society KJ-20 (to Toshihiro Furuta) and the Japan Society for the Promotion of Science KAKENHI Grant number 16K10332 (to Masayuki Yamaguchi).
Author information
Authors and Affiliations
Contributions
Study conception and design: TF, MY, MM, OA, HF. Acquisition of data: TF, MY. Analysis and interpretation of data: TF, MY. Drafting of manuscript: TF, MY. Critical revision: TF, MY, MM, OA, HF.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
The experimental protocol in the present study was approved by Committee for Ethics of Animal Experimentation of National Cancer Center, Tokyo, Japan.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Furuta, T., Yamaguchi, M., Minami, M. et al. Treatment margins in radiotherapy for liver tumors visualized as T2*-hypointense areas on SPIO-enhanced MRI at 9.4 T. Magn Reson Mater Phy 33, 701–712 (2020). https://doi.org/10.1007/s10334-020-00838-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10334-020-00838-4