Abstract
Objectives
Spin dephasing of the local magnetization in blood vessel networks can be described in the static dephasing regime (where diffusion effects may be ignored) by the established model of Yablonskiy and Haacke. However, for small capillary radii, diffusion phenomena for spin-bearing particles are not negligible.
Material and methods
In this work, we include diffusion effects for a set of randomly distributed capillaries and provide analytical expressions for the transverse relaxation times T2* and T2 in the strong collision approximation and the Gaussian approximation that relate MR signal properties with microstructural parameters such as the mean local capillary radius.
Results
Theoretical results are numerically validated with random walk simulations and are used to calculate capillary radius distribution maps for glioblastoma mouse brains at 9.4 T. For representative tumor regions, the capillary maps reveal a relative increase of mean radius for tumor tissue towards healthy brain tissue of \(128 \pm 23 \%\) (p < 0.001).
Conclusion
The presented method may be used to quantify angiogenesis or the effects of antiangiogenic therapy in tumors whose growth is associated with significant microvascular changes.





Similar content being viewed by others
Abbreviations
- CBV:
-
Cerebral blood volume
- CV:
-
Coefficient of variation
- DSC:
-
Dynamic susceptibility contrast
- GE:
-
Gradient echo
- Hct:
-
Hematocrit
- OEF:
-
Oxygen extraction fraction
- RD:
-
Radius distribution
- RO:
-
Random orientation
- RVM:
-
Random vessel distribution model
- SCA:
-
Strong collision approximation
- StDe:
-
Standard deviation
- SE:
-
Spin echo
- StEr:
-
Standard error
- SVM:
-
Single-vessel approximation model
- WFA:
-
Weak field approximation
References
Reichenbach JR, Schweser F, Serres B, Deistung A (2015) Quantitative susceptibility mapping: concepts and applications. Clin Neuroradiol 25:225–230
Liu S, Buch S, Chen Y, Choi HS, Dai Y, Habib C, Hu J, Jung JY, Luo Y, Utriainen D, Wang M, Wu D, Xia S, Haacke EM (2017) Susceptibility-weighted imaging: current status and future directions. NMR Biomed 30:e3552
Ogawa S, Lee TM, Kay AR, Tank DW (1990) Brain magnetic resonance imaging with contrast dependent on blood oxygenation. Proc Natl Acad Sci USA 87:9868–9872
Sehgal V, Delproposto Z, Haacke EM, Tong KA, Wycliffe N, Kido DK, Xu Y, Neelavalli J, Haddar D, Reichenbach JR (2005) Clinical applications of neuroimaging with susceptibility-weighted imaging. J Magn Reson Imaging 22:439–450
Deistung A, Schäfer A, Schweser F, Biedermann U, Turner R, Reichenbach JR (2013) Toward in vivo histology: A comparison of quantitative susceptibility mapping (QSM) with magnitude-, phase-, and R2*-imaging at ultra-high magnetic field strength. Neuroimage 65:299–314
Liu C (2010) Susceptibility tensor imaging. Magn Reson Med 63:1471–1477
Pauling L, Coryell CD (1936) The magnetic properties and structure of hemoglobin, oxyhemoglobin and carbonmonoxyhemoglobin. Proc Natl Acad Sci USA 22:210–216
Meyer EP, Ulmann-Schuler A, Staufenbiel M, Krucker T (2008) Altered morphology and 3D architecture of brain vasculature in a mouse model for Alzheimer’s disease. Proc Natl Acad Sci USA 105:3587–3592
Lauwers F, Cassot F, Lauwers-Cances V, Puwanarajah P, Duvernoy H (2008) Morphometry of the human cerebral cortex microcirculation: general characteristics and space-related profiles. Neuroimage 39:936–948
Yablonskiy DA, Haacke EM (1994) Theory of NMR signal behavior in magnetically inhomogeneous tissues: the static dephasing regime. Magn Reson Med 32:749–763
Yablonskiy DA, Sukstanskii AL, He X (2013) Blood oxygenation level-dependent (BOLD)-based techniques for the quantification of brain hemodynamic and metabolic properties-theoretical models and experimental approaches. NMR Biomed 26:963–986
Buxton RB (2013) The physics of functional magnetic resonance imaging (fMRI). Rep Prog Phys 76:096601
Grebenkov DS (2007) NMR survey of the reflected Brownian motion. Rev Mod Phys 79:1077–1137
Torrey HC (1956) Bloch equations with diffusion terms. Phys Rev 104:563–565
Jensen JH, Chandra R (2000) NMR relaxation in tissues with weak magnetic inhomogeneities. Magn Reson Med 44:144–156
Jensen JH, Chandra R, Ramani A, Lu H, Johnson G, Lee SP, Kaczynski K, Helpern JA (2006) Magnetic field correlation imaging. Magn Reson Med 55:1350–1361
Sukstanskii AL, Yablonskiy DA (2004) Gaussian approximation in the theory of MR signal formation in the presence of structure-specific magnetic field inhomogeneities. Effects of impermeable susceptibility inclusions. J Magn Reson 167:56–67
Ziener CH, Kampf T, Melkus G, Herold V, Weber T, Reents G, Jakob PM, Bauer WR (2007) Local frequency density of states around field inhomogeneities in magnetic resonance imaging: effects of diffusion. Phys Rev E 76:031915
Bauer WR, Nadler W, Bock M, Schad LR, Wacker C, Hartlep A, Ertl G (1999) The relationship between the BOLD-induced T2 and T2*: a theoretical approach for the vasculature of myocardium. Magn Reson Med 42:1004–1010
Dickson JD, Ash TW, Williams GB, Sukstanskii AL, Ansorge RE, Yablonskiy DA (2011) Quantitative phenomenological model of the BOLD contrast mechanism. J Magn Reson 212:17–25
Krogh A (1919) The supply of oxygen to the tissues and the regulation of the capillary circulation. J Physiol 52:457–474
Ziener CH, Kurz FT, Kampf T (2015) Free induction decay caused by a dipole field. Phys Rev E 91:032707
Kurz FT, Kampf T, Buschle LR, Heiland S, Schlemmer HP, Bendszus M, Ziener CH (2016) CPMG relaxation rate dispersion in dipole fields around capillaries. Magn Reson Imaging 34:875–888
Ziener CH, Kampf T, Reents G, Schlemmer HP, Bauer WR (2012) Spin dephasing in a magnetic dipole field. Phys Rev E 85:051908
Ziener CH, Kampf T, Melkus G, Jakob PM, Schlemmer HP, Bauer WR (2012) Signal evolution in the local magnetic field of a capillary—analogy to the damped driven harmonic oscillator. Magn Reson Imaging 30:540–553
Kurz FT, Buschle LR, Kampf T, Zhang K, Schlemmer HP, Heiland S, Bendszus M, Ziener CH (2016) Spin dephasing in a magnetic dipole field around large capillaries: approximative and exact results. J Magn Reson 273:83–97
Harrison RV, Harel N, Panesar J, Mount RJ (2002) Blood capillary distribution correlates with hemodynamic-based functional imaging in cerebral cortex. Cereb Cortex 12:225–233
Bauer WR, Nadler W, Bock M, Schad LR, Wacker C, Hartlep A, Ertl G (1999) Theory of the BOLD effect in the capillary region: an analytical approach for the determination of T2 in the capillary network of myocardium. Magn Reson Med 41:51–62
Breckwoldt MO, Bode J, Kurz FT, Hoffmann A, Ochs K, Ott M, Deumelandt K, Krüwel T, Schwarz D, Fischer M, Helluy X, Milford D, Kirschbaum K, Solecki G, Chiblak S, Abdollahi A, Winkler F, Wick W, Platten M, Heiland S, Bendszus M, Tews B (2016) Correlated magnetic resonance imaging and ultramicroscopy (MR-UM) is a tool kit to assess the dynamics of glioma angiogenesis. eLife 5:e11712
Spees WM, Yablonskiy DA, Oswood MC, Ackerman JJH (2001) Water proton MR properties of human blood at 1.5 Tesla: magnetic susceptibility, T1, T2, T2*, and non-Lorentzian signal behavior. Magn Reson Med 45:533–542
Brown RW, Haacke EM, Cheng YC, Thompson MR, Venkatesan R (2014) Magnetic resonance imaging: physical principles and sequence design. John Wiley & Sons, Hoboken
Boxerman JL, Hamberg LM, Rosen BR, Weisskoff RM (1995) MR contrast due to intravascular magnetic susceptibility perturbations. Magn Reson Med 34:555–566
Purves WK, Orians GH, Sadava D, Heller HC (2003) Life: the science of biology: volume III: plants and animals. Macmillan, Basingstoke
He X, Zhu M, Yablonskiy DA (2008) Validation of oxygen extraction fraction measurement by qBOLD technique. Magn Reson Med 60:882–888
Sukstanskii AL, Yablonskiy DA (2003) Gaussian approximation in the theory of MR signal formation in the presence of structure-specific magnetic field inhomogeneities. J Magn Reson 163:236–247
Ziener CH, Kampf T, Jakob PM, Bauer WR (2010) Diffusion effects on the CPMG relaxation rate in a dipolar field. J Magn Reson 202:38–42
Chugh BP, Lerch JP, Lisa XY, Pienkowski M, Harrison RV, Henkelman RM, Sled JG (2009) Measurement of cerebral blood volume in mouse brain regions using micro-computed tomography. Neuroimage 47:1312–1318
Ziener CH, Kampf T, Kurz FT (2015) Diffusion propagators for hindered diffusion in open geometries. Concepts Magn Reson A 44:150–159
Kurz FT, Kampf T, Buschle LR, Schlemmer HP, Bendszus M, Heiland S, Ziener CH (2016) Generalized moment analysis of magnetic field correlations for accumulations of spherical and cylindrical magnetic pertubers. Front Phys 4:46
Kiselev VG, Posse S (1998) Analytical theory of susceptibility induced NMR signal dephasing in a cerebrovascular network. Phys Rev Lett 81:5696–5699
Kiselev VG, Posse S (1999) Analytical model of susceptibility-induced MR signal dephasing: effect of diffusion in a microvascular network. Magn Reson Med 41:499–509
Dattagupta S, Blume M (1974) Stochastic theory of line shape. I. Nonsecular effects in the strong-collision model. Phys Rev B 10:4540–4550
Bauer WR, Nadler W, Bock M, Schad LR, Wacker C, Hartlep A, Ertl G (1999) Theory of coherent and incoherent nuclear spin dephasing in the heart. Phys Rev Lett 83:4215–4218
Kurz FT, Ziener CH, Rückl M, Hahn A, Sturm VJF, Zhang K, Buschle LR, Bendszus M, Heiland S, Schlemmer HP, Bauer WR, Kampf T (2017) The influence of spatial patterns of capillary networks on transverse relaxation. Magn Reson Imaging 40:31–47
Mooney CZ (1997) Monte Carlo simulation. Sage Publications, Thousand Oaks
Hall MG, Alexander DC (2009) Convergence and parameter choice for Monte-Carlo simulations of diffusion MRI. IEEE Trans Med Imaging 28:1354–1364
Knuth DE (2013) Art of computer programming. Addison-Wesley, Boston
von Baumgarten L, Brucker D, Tirniceru A, Kienast Y, Grau S, Burgold S, Herms J, Winkler F (2011) Bevacizumab has differential and dose-dependent effects on glioma blood vessels and tumor cells. Clin Cancer Res 17:6192–6205
Hennig J, Nauerth A, Friedburg H (1986) RARE imaging: a fast imaging method for clinical MR. Magn Reson Med 3:823–833
Stables LA, Kennan RP, Gore JC (1998) Asymmetric spin-echo imaging of magnetically inhomogeneous systems: theory, experiment, and numerical studies. Magn Reson Med 40:432–442
Weisskoff RM, Zuo CS, Boxerman JL, Rosen BR (1994) Microscopic susceptibility variation and transverse relaxation: theory and experiment. Magn Reson Med 31:601–610
Cassot F, Lauwers F, Fouard C, Prohaska S, Lauwers-Cances V (2006) A novel three-dimensional computer-assisted method for a quantitative study of microvascular networks of the human cerebral cortex. Microcirculation 13:1–18
Duvernoy HM, Delon S, Vannson JL (1981) Cortical blood vessels of the human brain. Brain Res Bull 7:519–579
Duvernoy H, Delon S, Vannson JL (1983) The vascularization of the human cerebellar cortex. Brain Res Bull 11:419–480
van Zijl PCM, Eleff SM, Ulatowski JA, Ulug AM, Traystman RJ, Kauppinen RA (1998) Quantitative assessment of blood flow, blood volume and blood oxygenation effects in functional magnetic resonance imaging. Nat Med 4:159–167
Martindale J, Kennerley AJ, Johnston D, Zheng Y, Mayhew JE (2008) Theory and generalization of Monte Carlo models of the BOLD signal source. Magn Reson Med 59:607–618
Grandin CB, Bol A, Smith AM, Michel C, Cosnard G (2005) Absolute CBF and CBV measurements by MRI bolus tracking before and after acetazolamide challenge: repeatability and comparison with PET in humans. Neuroimage 26:525–535
Pathak AP, Kim E, Zhang J, Jones MV (2011) Three-dimensional imaging of the mouse neurovasculature with magnetic resonance microscopy. PLoS ONE 6:e22643
Guilfoyle DN, Dyakin VV, O’Shea J, Pell GS, Helpern JA (2003) Quantitative measurements of proton spin-lattice (T1) and spin-spin (T2) relaxation times in the mouse brain at 7.0 T. Magn Reson Med 49:576–580
Le Bihan D (1995) Molecular diffusion, tissue microdynamics and microstructure. NMR Biomed 8:375–386
Troprés I, Lamalle L, Peoc’h M, Farion R, Usson Y, Decorps M, Remy C (2004) In vivo assessment of tumoral angiogenesis. Magn Reson Med 51:533–541
Valable S, Lemasson B, Farion R, Beaumont M, Segebarth C, Remy C, Barbier EL (2008) Assessment of blood volume, vessel size, and the expression of angiogenic factors in two rat glioma models: a longitudinal in vivo and ex vivo study. NMR Biomed 21:1043–1056
Ziener CH, Bauer WR, Jakob PM (2005) Transverse relaxation of cells labeled with magnetic nanoparticles. Magn Reson Med 54:702–706
Buschle LR, Kurz FT, Kampf T, Triphan SMF, Schlemmer HP, Ziener CH (2015) Diffusion-mediated dephasing in the dipole field around a single spherical magnetic object. Magn Reson Imaging 33:1126–1145
Mueller J, Raisi H, Rausch V, Peccerella T, Simons D, Ziener CH, Schlemmer HP, Seitz HK, Waldburger N, Longerich T, Straub BK, Mueller S (2017) Sensitive and non-invasive assessment of hepatocellular iron using a novel room-temperature susceptometer. J Hepatol 67:535–542
Winkler F, Kienast Y, Fuhrmann M, Von Baumgarten L, Burgold S, Mitteregger G, Kretzschmar H, Herms J (2009) Imaging glioma cell invasion in vivo reveals mechanisms of dissemination and peritumoral angiogenesis. Glia 57:1306–1315
Farrar CT, Kamoun WS, Ley CD, Kim YR, Kwon SJ, Dai G, Rosen BR, di Tomaso E, Jain RK, Sorensen AG (2010) In vivo validation of MRI vessel caliber index measurement methods with intravital optical microscopy in a U87 mouse brain tumor model. Neuro Oncol 12(4):341–350
Quail DF, Joyce JA (2013) Microenvironmental regulation of tumor progression and metastasis. Nat Med 19:1432–1437
Linkous AG, Yazlovitskaya EM (2011) Angiogenesis in glioblastoma multiforme: navigating the maze. Anticancer Agents Med Chem 11:712–718
Cipolla MJ (2009) The cerebral circulation. Morgan & Claypool Life Sciences, San Rafael
Fukunaga M, Li TQ, van Gelderen P, de Zwart JA, Shmueli K, Yao B, Maric Lee J, Aronova MA, Zhang G, Leapman RD, Schenck JF, Merkle H, Duyn JH (2010) Layer-specific variation of iron content in cerebral cortex as a source of MRI contrast. Proc Natl Acad Sci USA 107:3834–3839
Hocq A, Brouette N, Saussez S, Luhmer M, Gillis P, Gossuin Y (2009) Variable-field relaxometry of iron-containing human tissues: a preliminary study. Contrast Media Mol Imaging 4:157–164
Yablonskiy DA (1998) Quantitation of intrinsic magnetic susceptibility-related effects in a tissue matrix. Phantom study. Magn Reson Med 39:417–428
Domsch S, Mie MB, Wenz F, Schad LR (2014) Non-invasive multiparametric qBOLD approach for robust mapping of the oxygen extraction fraction. Z Med Phys 24:231–242
Christen T, Bolar DS, Zaharchuk G (2013) Imaging brain oxygenation with MRI using blood oxygenation approaches: methods, validation, and clinical applications. AJNR Am J Neuroradiol 34:1113–1123
Troprés I, Grimault S, Vaeth A, Grillon E, Julien C, Payen JF, Lamalle L, Decorps M (2001) Vessel size imaging. Magn Reson Med 45(3):397–408
Wen J, Cross AH, Yablonskiy DA (2015) On the role of physiological fluctuations in quantitative gradient echo MRI: implications for GEPCI, QSM, and SWI. Magn Reson Med 73:195–203
Ulrich X, Yablonskiy DA (2016) Separation of cellular and BOLD contributions to T2* signal relaxation. Magn Reson Med 75:606–615
Kennan RP, Zhong J, Gore JC (1994) Intravascular susceptibility contrast mechanisms in tissues. Magn Reson Med 31:9–21
Buschle LR, Kurz FT, Kampf T, Wagner WL, Duerr J, Stiller W, Konietzke P, Wünnemann F, Mall MA, Wielpütz MO, Schlemmer HP, Ziener CH (2017) Dephasing and diffusion on the alveolar surface. Phys Rev E 95:022415
Emblem KE, Farrar CT, Gerstner ER, Batchelor TT, Borra RJH, Rosen BR, Sorensen AG, Jain RK (2014) Vessel calibre—a potential MRI biomarker of tumour response in clinical trials. Nat Rev Clin Oncol 11:566–584
Emblem KE, Mouridsen K, Bjornerud A, Farrar CT, Jennings D, Borra RJH, Wen PY, Ivy P, Batchelor TT, Rosen BR, Jain RK, Sorensen AG (2013) Vessel architectural imaging identifies cancer patient responders to anti-angiogenic therapy. Nat med 19(9):1178–1183
Dennie J, Mandeville JB, Boxerman JL, Packard SD, Rosen BR, Weisskoff RM (1998) NMR imaging of changes in vascular morphology due to tumor angiogenesis. Magn Reson Med 40(6):793–799
Troprés I, Pannetier N, Grand S, Lemasson B, Moisan A, Péoc’h M, Rémy C, Barbier EL (2015) Imaging the microvessel caliber and density: principles and applications of microvascular MRI. Magn Reson Med 73:325–341
Stadlbauer A, Zimmermann M, Heinz G, Oberndorfer S, Doerfler A, Buchfelder M, Rössler K (2017) Magnetic resonance imaging biomarkers for clinical routine assessment of microvascular architecture in glioma. J Cereb Blood Flow Metab 37:632–643
Jochimsen TH, Moller HE (2008) Increasing specificity in functional magnetic resonance imaging by estimation of vessel size based on changes in blood oxygenation. Neuroimage 40:228–236
Pannetier NA, Sohlin M, Christen T, Schad L, Schuff N (2014) Numerical modeling of susceptibility-related MR signal dephasing with vessel size measurement: phantom validation at 3T. Magn Reson Med 72:646–658
Kording F, Weidensteiner C, Zwick S, Osterberg N, Weyerbock A, Staszewski O, Elverfeldt D, Reichardt W (2014) Simultaneous assessment of vessel size index, relative blood volume, and vessel permeability in a mouse brain tumor model using a combined spin echo gradient echo echo-planar imaging sequence and viable tumor analysis. J Magn Reson Imaging 40:1310–1318
Bonekamp D, Mouridsen K, Radbruch A, Kurz FT, Eidel O, Wick A, Schlemmer HP, Wick W, Bendszus M, Østergaard L (2017) Kickingereder P (2017) Assessment of tumor oxygenation and its impact on treatment response in bevacizumab-treated recurrent glioblastoma. J Cereb Blood Flow Metab 37:485–494
Ziener CH, Glutsch S, Jakob PM, Bauer WR (2009) Spin dephasing in the dipole field around capillaries and cells: numerical solution. Phys Rev E 80:046701
Ziener CH, Bauer WR, Jakob PM (2005) Frequency distribution and signal formation around a vessel. Magn Reson Mater Phy 18:225–230
Ziener CH, Rückl M, Kampf T, Bauer WR, Schlemmer HP (2012) Mathieu functions for purely imaginary parameters. J Comput Appl Math 236:4513–4524
Ziener CH, Kurz FT, Buschle LR, Kampf T (2015) Orthogonality, Lommel integrals and cross product zeros of linear combinations of Bessel functions. SpringerPlus 4:390
Moiseyev N (2011) Non-Hermitian quantum mechanics. Cambridge University Press, Cambridge
Bagarello F, Gazeau JP, Szafraniec FH, Znojil M (2015) Non-selfadjoint operators in quantum physics: mathematical aspects. John Wiley & Sons, Hoboken
Herberthson M, Özarslan E, Knutsson H, Westin CF (2017) Dynamics of local magnetization in the eigenbasis of the Bloch-Torrey operator. J Chem Phys 146:12401
Seeger A (1979) The determination of the diffusion coefficient of positive muons from the transverse depolarization in type-II superconductors. Phys Lett A 74:259–262
Seeger A (1997) Transverse spin relaxation of spin carriers diffusing in spatially periodic magnetic fields. Hyperfine Interact 105:151–159
Stoller SD, Happer W, Dyson FJ (1991) Transverse spin relaxation in inhomogeneous magnetic fields. Phys Rev A 44:7459–7477
Bender CM, Brody DC, Jones HF (2003) Must a hamiltonian be hermitian? Am J Phys 71:1095–1102
Gohberg IC, Krein MG (1969) Introduction to the theory of linear nonselfadjoint operators. American Mathematical Society, Providence
Widder DV (1946) The Laplace transformation. Princeton University Press, Princeton
Kurz FT, Kampf T, Heiland S, Bendszus M, Schlemmer HP, Ziener CH (2014) Theoretical model of the single spin-echo relaxation time for spherical magnetic perturbers. Magn Reson Med 71:1888–1895
Kurz FT, Kampf T, Buschle LR, Schlemmer HP, Heiland S, Bendszus M, Ziener CH (2015) Microstructural analysis of peripheral lung tissue through CPMG inter-echo time R2 dispersion. PLoS One 10:e0141894
Ziener CH, Kampf T, Melkus G, Jakob PM, Bauer WR (2007) Scaling laws for transverse relaxation times. J Magn Reson 184:169–175
Ziener CH, Kampf T, Herold V, Jakob PM, Bauer WR, Nadler W (2008) Frequency autocorrelation function of stochastically fluctuating fields caused by specific magnetic field inhomogeneities. J Chem Phys 129:014507
Bauer WR, Ziener CH, Jakob PM (2005) Non-Gaussian spin dephasing. Phys Rev A 71:053412
Acknowledgements
This work was supported by grants from the Deutsche Forschungsgemeinschaft (contract grant number: DFG ZI 1295/2-1 and DFG KU 3555/1-1). L. R. Buschle was supported by a scholarship of the German Academic Scholarship Foundation (Studienstiftung des deutschen Volkes). F. T. Kurz was supported by a postdoctoral fellowship from the medical faculty of Heidelberg University and the Hoffmann-Klose foundation of Heidelberg University.
Author information
Authors and Affiliations
Contributions
This work was carried out by the all authors in collaboration. LRB and FTK designed the research; LRB, CHZ, KZ, VJFS, AH, GS and FTK performed the research; LRB contributed numerical tools; and LRB, CHZ, TK, HS, FW, MB, SH, HPS and FTK wrote the paper.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Ethical approval
All applicable international, national and/or institutional guidelines for the care and use of animals were followed. All procedures performed in studies involving animals were in accordance with the ethical standards of the institution or practice at which the studies were conducted.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Appendices
Appendix A: Single-vessel approximation model (SVM)
In this appendix, the random vessel distribution model (RVM) is compared to the single-vessel approximation model (SVM). Static dephasing limit, an exact solution of the Bloch-Torrey equation in the SVM and the SCA in the SVM were summarized and compared to the obtained results in the RVM.
Geometrical model
The Bloch-Torrey equation can be solved for uniformly arranged capillaries by applying Krogh’s capillary supply model [21] to reduce the vessel network geometry to one single vessel. In this model, only one single capillary with radius R is considered, which is surrounded by a second concentric cylinder, or Krogh cylinder, that accounts for the surrounding tissue. The outer radius \(R_\text {D}\) is chosen such that the capillary blood volume \(\eta \) corresponds to:
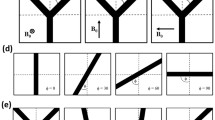
Reflecting boundary conditions are chosen at the surface of the Krogh cylinder (see e.g. Fig. 1 in [90]). A schematic illustration of the SVM is shown in Fig. 1c.
This simplistic geometrical model provides an exact solution of the Bloch-Torrey Eq. (1) for spin dephasing around capillaries, as demonstrated recently (see also below and [22, 24, 26]).
Static dephasing regime
In the static dephasing regime (index 0), where diffusion effects are neglected, the free induction decay in the SVM can be found as the signal around a single blood vessel (see [18] and Eq. (36) in [10]):
where \(J_0(x\delta \omega t)\) denotes the Bessel function of first kind with an index of zero. The function h(x) can be expressed in terms of the hypergeometric function as given in Eq. (26). Equation (40) follows from Eq. (29) in [25] and allows calculating the mathematical limits \(\eta \rightarrow 0\) and \(\eta \rightarrow 1\).
The frequency distribution \(p(\omega )\) is defined as the Fourier transform of the free induction decay:
Since \(M(0) = 1\) [see Eq. (3)], \(p(\omega )\) is normalized to:
The frequency distribution in the static dephasing limit for the SVM was found as [18]:
Evidently, \(p(\omega )\) exhibits two prominent peaks at \(\omega = \pm \eta \delta \omega \). The frequency distribution from Eq. (43) possesses a local minimum at \(\omega = 0\), whereas \(p(\omega )\) in the RVM peaks globally at \(\omega = 0\) (as shown in the blue lines in Fig. 6). The mean relaxation time approach to determine the relaxation rate \(R_2^{*}\) [see Eq. (4)], however, translates into the Fourier domain as follows [91]:
Thus, it follows that the relaxation rate for the SVM in the static dephasing regime is twice as large as that for the RVM, while both frequency distributions exhibit a similar lineshape, i.e. both distributions have the same width:
In the limit of static dephasing, Yablonskiy and Haacke could connect the free induction decay of N randomly distributed capillaries, \(M_{0,\text {RVM}}\), with the free induction decay around one single blood vessel, \(M_{0,\text {SVM}}\), as in [10]:
where \(M_{0,\text {SVM}}\) is given in Eq. (39). In the statistical limit \(N\rightarrow \infty \), the gradient echo signal in the RVM is then given in Eq. (10).
Exact solution of the Bloch-Torrey equation in the SVM
An analytical solution of the local magnetization and the free induction decay in the SVM can be obtained with an eigenfunction expansion of the Bloch-Torrey equation [22, 24].
The eigenvalues \(\lambda _{nm}\) in the SVM are, in general, complex and obey the following condition:
Here \(J_{k_m}\) and \(Y_{k_m}\) are Bessel functions of the first and second kind and \(J^{\prime }_{k_m}\) and \(Y^{\prime }_{k_m}\) are derivations of Bessel functions, respectively. The index \(k_m\) relates to the interplay between diffusion and susceptibility effects, since:
where \(a_{2m}\) denotes the characteristic values of the Mathieu functions [92]. The expansion coefficients \(d_{nm}\) are given as:
with \(s^{\prime }_{1,k_m}\) denoting the first derivative of the Lommel function \(s_{1,k_m}\) (see also [93]). The symbol \(A_0^{(2m)}\) represents the first Fourier coefficient of the even \(\pi \)-periodic Mathieu functions \(\text {ce}_{2m}\) and also depends on the parameter \(\delta \omega R^2/D\). This expansion uses angular eigenvalues \(\kappa _m\) and associated eigenvectors with an index m, as well as radial eigenvalues \(\lambda_{nm}\) and associated eigenvectors with indices m and n. The indices n and m are therefore analogues of the radial and angular quantum numbers in quantum mechanics. The total magnetization is then given as sum over all eigenfunctions and corresponding eigenvalues as given in Eq. (47). One could argue against such an expansion since the Bloch-Torrey operator, \(D\Delta -\mathrm {i}\omega \), is non-hermitian. However, an operator does not necessarily need to be hermitian to allow for an eigenfunction expansion as e.g. exemplified in [94, 95]. Furthermore, Herberthson et al. provided proof that the eigenfunctions of the Bloch-Torrey operator for a linear field gradient are self-dual [96]. This proof can easily be generalized for arbitrary magnetic field inhomogeneities for reflecting boundary conditions:
Lemma 1
Suppose u and v are eigenfunctions of the operator \(\Delta +z(\mathbf {r})\) with the eigenvalues \(\upmu \) and \(\lambda \), respectively, which fulfill reflecting boundary conditions at the surface \(\partial \Omega \) of the volume \(\Omega \). The function \(z(\mathbf {r})\in \mathbb {C}\) is an arbitrary complex function. Then, the eigenfunctions are self-dual, e.g. if \(\upmu \ne \lambda \), then\(\int _\Omega \mathrm {d}\Omega \, uv = 0\).
Proof
Suppose \(\upmu \ne \lambda \).
where the second term vanishes due to the reflecting boundary conditions and Green’s identity was applied. Hereby, \(\hat{n}\) denotes a unity vector that is orthogonal to the surface \(\partial \Omega \) of the volume \(\Omega \), \(\mathrm {d}\Omega \) symbolizes the volume integration and \(\mathrm {d}s\) symbolizes the integration over the surface \(\partial \Omega \). Finally, one obtains:
Thus, the statement follows. \(\square \)
In the special case \(z(\mathbf {r}) \propto -\mathrm {i}\omega (\mathbf {r})\), where the function z takes purely imaginary values, the eigenfunctions of the Bloch-Torrey operator are self-dual. This agrees with Eq. (4) in [92] and Eqs. (9) and (12) in [24]. In addition, an actual eigenfunction expansion for the Bloch-Torrey equation has been successfully developed in several publications, e.g. [97,98,99,100]. A more detailed account is given in [101].
Frequency distributions for \(\eta = 0.05\) and \(\delta \omega = 1000/\text {s}\), which correspond to \(B_0=9.4\, \text {T}\) and \(D=1\,\upmu \text {m}^2/\text {ms}\) for different capillary radii. The solid lines present the strong collision approximation (SCA) of the randomly positioned capillary model obtained from Eq. (82), and exact solutions in the single-vessel approximation are shown as dashed lines [see Eq. (47)]. In the static dephasing limit, both distributions exhibit the same width [see Eq. (45)]. The frequency distribution in the SVM, however, possesses a local minimum at \(\omega = 0\) for large capillary radii, whereas the RVM contains a global maximum at \(\omega = 0\). This difference gives different estimations of the relaxation rate \(R_2^{*}\), as shown in Eq. (44)
According to Eq. (4), the relaxation rate in this model can be found as:
Strong collision approximation (SCA)
In the static dephasing limit, the free induction decay is given in Eq. (39); its Laplace transform then follows as:
where the \(\hat{}\) symbol denotes the Laplace transform of a quantity as defined in Eq. (72) in “Appendix C”. We then find the SCA relaxation rate as [25]:
In the SVM, the correlation time \(\tau \), as given in Eq. (73) in “Appendix C”, can be obtained as [18]:
and the spin echo relaxation rate is determined by [19]:
with the gradient echo relaxation rate \(R_2^{*}\) from Eq. (56) and the correlation time \(\tau \) from Eq. (57). Thus, the spin echo relaxation rate in the SCA reads:
Appendix B: Weak field approximation
In analogy to Jensen et al. [15], the correlation function for a randomized distribution of permeable capillaries is given as [18, 38, 106]:
where the free diffusion of spin-bearing particles was assumed, which is a good approximation for small capillary blood volume fractions \(\eta \). The correlation function at the initial time point \(t = 0\) takes the value: \(K(0) = \langle \omega ^2(\mathbf {r}) \rangle \). Using the local Larmor frequency given in Eq. (7), we can simplify K(t) to:
where \(\hat{x}\) is the unit vector in the spatial x direction and a random distribution of capillaries in a plane is assumed. This approximation, however, allows for an overlap of capillary positions. Yet, for low capillary blood volume fractions \(\eta \), the occurrences of capillary overlaps are very scarce and, thus, can be neglected. With the representation of the diffusion propagator in Fourier space
we find
where \(\phi \) is the angle between the position vector \(\mathbf {r}\) and the transverse component of the magnetic field (see Fig. 1) and \(\vartheta \) denotes the angle between the reciprocal vector \(\mathbf {k}\) and the magnetic field. Evaluating the angular part \(\phi \) of the integral, we find:
where \(J_1\) and \(J_2\) are Bessel functions of the first kind. By evaluating the integrals over \(\vartheta \) and k, the correlation function follows as:
where \(I_0\) and \(I_1\) are modified Bessel functions of the first kind. The prefactor \(\eta \delta \omega ^2/2 = \langle \omega ^2 \rangle \) equals the width of the frequency distribution as demonstrated below [see Eq. (45)]. For very long or short times, the correlation function can be approximated as:
According to [15], the weak field approximation (WFA) is valid for
Since \(R_2^{*} \approx \frac{\eta }{2}\tau \delta \omega ^2\) in the motional narrowing regime, the WFA is valid for \(\eta \frac{R^4}{D^2}\delta \omega ^2 < 3.72\). Non-Gaussian spin behaviour was analyzed in [107].
In Fig. 7, the calculated correlation function is compared with the correlation function of Sukstanskii and Yablonskiy as obtained in the RVM for impermeable vessel walls [17, 38]:
where \(J_2^{\prime }\) is the first derivative of the Bessel functions of the first kind with index 2 and \(Y_2^{\prime }\) is the first derivative of the Bessel functions of the second kind with index 2.
Comparison of the correlation function in the RVM for permeable vessel walls, given in Eq. (67), with the correlation function in the RVM for impermeable vessels (yellow line), given in Eq. (70) and the correlation function in the SVM (see Ref. [106], red line). All functions coincide for short times. The blue dotted line shows the monoexponential approximation of the correlation function with the correlation time given in Eq. (76)
As shown in Fig. 7, the correlation function in the RVM for impermeable vessels is similar to the correlation function in the RVM for permeable vessels. Especially, both curves exhibit the same asymptotic behaviour for long times that is shown in Eq. (68).
Random walk simulations were performed to validate the correlation functions. Therefore, 100,000 random walk trajectories were simulated in a volume with 17,500 randomly distributed cylindrical vessels with capillary volume fraction \(\eta =0.05\). The obtained correlation function is compared in Fig. 7 with the correlation functions in the SVM and RVM for permeable and impermeable vessels.
Appendix C: strong collision approximation (SCA) in the random vessel distribution model (RVM)
In the SCA, the diffusion operator in the Bloch-Torrey equation is replaced by a simpler Markov operator. In a Markov process, the diffusion transition rate between different positions is only dependent on the equilibrium probability of the final state. One of the main results of the SCA condenses in the connection between the Laplace transform of the free induction decay with the Laplace transform of the static dephasing free induction decay and the correlation time \(\tau \) [see Eq. (20) and [28]]:
where the Laplace transform is defined as:
Lerch’s uniqueness theorem guarantees the uniqueness of the inverse Laplace transform of \(\hat{M}(s)\), even if the function M(t) is only piecewise continuous [102]. With the strong collision assumption, the correlation function exhibits monoexponential decay with the correlation time \(\tau \) of the form [107]:
The correlation time \(\tau \) can then be obtained with the least squares method by minimizing the functional difference between the correlation function K(t) with its monoexponential approximation [see Eq. (19)]. For permeable vessel boundaries, we obtained with the correlation function K(t) in Eq. (67) the correlation time \(\tau _\text {per}\) as a solution of the equation:
where G denotes Meijers G-function. The correlation time \(\tau _{\text {per}}\) can then obtained as
For impermeable vessels, the correlation time \(\tau \) is also defined by minimizing the expression given in Eq. (19):
where K(t) is given in Eq. (70). Since impermeable vessel walls provide a more realistic tissue model than permeable vessels walls, the correlation time of impermeable vessels is used for the SCA in the RVM.
Details about the derivation of Eq. (71) are presented in appendix A in Ref. [18], or in [64, 103, 104]. For the static dephasing magnetization in the RVM given in Eq. (28), the Laplace transform follows as:
where \(\mathrm {erf}(z_0,z_1) = \mathrm {erf}(z_1)- \mathrm {erf}(z_0)\). When inserting the Laplace transform of the RVM static dephasing free induction decay into Eq. (71), one finds:
The monoexponential relaxation rate \(R_2^{*}\) is defined in Eq. (4):
Inserting the definition of the Laplace transform given in Eq. (72), one finds Eq. (20). Finally, the gradient echo relaxation rate can be obtained as:
A Taylor expansion with respect to the capillary blood volume fraction \(\eta \) leads to Eq. (30). Both gradient echo relaxation rate \(R_2^{*}\) and spin echo relaxation rate \(R_2\) are shown in Figs. 8 and 9 for different magnetic field strengths \(B_0\), for a blood volume fraction of \(\eta = 0.05\), a dipole field strength of \(\delta \omega \approx 100 \frac{1}{\text {s} \text {T}} B_0\) in accordance with Eq. (8) and a diffusion coefficient of \(D=1\,\upmu \text {m}^2 \text {ms}^{-1}\) in accordance with [41]. A change of the blood volume fraction would linearly scale the ordinates in Figs. 8 and 9, while the abscissas show a dependence on the product \(\delta \omega R^2/D\) [105].
Gradient echo relaxation rate \(R_2^{*}\) for different magnetic field strength \(B_0\). The static dephasing regime is valid for large vessel radii and the weak field approximation in the opposite limit for small vessel radii. The strong collision approximation (SCA) agrees very well with random walk simulations for arbitrary vessel radii
Spin echo relaxation rate \(R_2\) for different magnetic field strengths \(B_0\). The slow diffusion approximation is valid for large vessel radii and its accuracy increases with increasing magnetic field strengths. The weak field approximation is valid in the opposite limit for small vessel radii and small magnetic field strengths. The strong collision approximation (SCA) can only qualitatively describe the shape of the \(R_2\) simulation data points
With the Laplace transform of the free induction decay, \(\hat{M}_{\text {RVM}}(s)\), the frequency distribution follows as:
In Fig. 6, the frequency distribution is shown for different capillary radii; evidently, with increasing capillary radius, the frequency distribution broadens.
So far, the gradient echo signal was analyzed for a fixed angle \(\theta \) between the main magnetic field and vessel orientation. An averaging over all possible orientations (index RO) is therefore a natural step to account for the intrinsic inhomogeneities of blood vessel networks. In this limit, Yablonskiy and Haacke obtained an expression for the static dephasing magnetization given in Eq. (33). Using a similar procedure as above, we can obtain the Laplace transform
and, eventually, the SCA relaxation rate \(\overline{R_2^{*}}\) as:
A Taylor expansion in order of \(\eta \) again leads to Eq. (34).
Rights and permissions
About this article
Cite this article
Buschle, L.R., Ziener, C.H., Zhang, K. et al. Vessel radius mapping in an extended model of transverse relaxation. Magn Reson Mater Phy 31, 531–551 (2018). https://doi.org/10.1007/s10334-018-0677-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10334-018-0677-9