Abstract
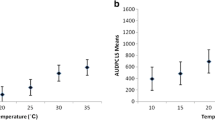
Ralstonia solanacearum, the causal agent of bacterial wilt of tomato, grows in infected plants and migrates from the roots into the soil. We investigated the effectiveness of bacterial wilt-resistant tomato rootstock in reducing the migration of R. solanacearum from susceptible scions into the soil. Rootstock stems were either 3–5 cm tall (low-grafted, LG) or ≥ 10 cm tall (high-grafted, HG). After inoculation of scions of the susceptible cultivar (SC) with R. solanacearum below the first flower, there was no difference in disease progression among LG, HG, and ungrafted SC plants, and plants had wilted by 2 weeks. However, the rate of detection of R. solanacearum in the soil of wilted plants was reduced by grafting. The size of the R. solanacearum population in the soil of fully wilted plants increased in the order of HG < LG < SC. These results show that grafting onto resistant rootstock strongly suppressed the migration of R. solanacearum into the soil by the time of full wilting, and the effect was stronger with a longer rootstock. Migration of R. solanacearum into soil increased with increasing disease severity in SC, LG and HG. These facts suggest that early uprooting of slightly infected plants could control the spread of the bacteria into the soil.



Similar content being viewed by others
References
Álvarez B, Biosca EG, López MM (2010) On the life of Ralstonia solanacearum, a destructive bacterial plant pathogen. In: Méndez-Vilas A (ed) Current research, technology and education topics in applied microbiology and microbial biotechnology, vol 1. Formatex, Badajoz, pp 267–279
Bae C, Han SW, Song YR, Kim BY, Lee HJ, Lee JM, Yeam I, Heu S, Oh CS (2015) Infection processes of xylem-colonizing pathogenic bacteria: possible explanations for the scarcity of qualitative disease resistance genes against them in crops. Theor Appl Genet 128:1219–1229
Denny TP (2006) Plant pathogenic Ralstonia species. In: Gnanamanickam SS (ed) Plant-associated bacteria. Springer, Dordrecht, pp 573–644
Elphinstone JG, Henessy J, Wilson JK, Stead D (1996) Sensitivity of different methods for the detection of Ralstonia solanacearum in potato tuber extracts. Bull OEPP/EPPO Bull 26:663–678
Goto M (1992) Fundamentals of bacterial plant pathology. Academic Press, New York, p 342
Grimault V, Prior P (1994) Grafting tomato cultivars resistant or susceptible to bacterial wilt: analysis of resistant mechanisms. J Phytopathol 141:330–334
Hayward AC (1991) Biology and epidemiology of bacterial wilt caused by Pseudomonas solanacearum. Annu Rev Phytopathol 29:65–87
Hikichi Y, Nakazawa-Nasu Y, Kitanosono S, Suzuki K, Okuno T (1999) The behavior of lux-marked Ralstonia solanacearum in grafted tomato cultivars resistant or susceptible to bacterial wilt. Ann Phytopathol Soc Jpn 65:597–603
Inoue Y, Nakaho K (2014) Sensitive quantitative detection of Ralstonia solanacearum in soil by the most probable number-polymerase chain reaction (MPN-PCR) method. Appl Microbiol Biotechnol 98:4169–4177
Khah EM, Kakava A, Mavromatis A, Chachalis D, Goulas C (2006) Effect of grafting on growth and yield of tomato (Lycopersicon esculentum Mill.) in greenhouse and open-field. J Appl Hort 8:3–7
Milling A, Babujee L, Allen C (2011) Ralstonia solanacearum extracellular polysaccharide is a specific elicitor of defense responses in wilt-resistant tomato plants. PLoS One 6:e15853
Nakaho K (1997a) Distribution and multiplication of Ralstonia solanacearum (synonym Pseudomonas solanacearum) in tomato plants of resistant rootstock cultivar LS-89 and susceptible Ponderosa. Ann Phytopathol Soc Jpn 63:83–88
Nakaho K (1997b) Distribution and multiplication of Ralstonia solanacearum in stem-inoculated tomato rootstock cultivar LS-89 resistant to bacterial wilt. Ann Phytopathol Soc Jpn 63:341–344
Nakaho K, Allen C (2009) A pectinase-deficient Ralstonia solanacearum strain induces reduced and delayed structural defences in tomato xylem. J Phytopathol 157:228–234
Nakaho K, Takaya S, Sumida Y (1996) Conditions that increase latent infection of grafted or non-grafted tomatoes with Pseudomonas solanacearum. Ann Phytopathol Soc Jpn 62:234–239
Nakaho K, Hibino H, Miyagawa H (2000) Possible mechanisms limiting movement of Ralstonia solanacearum in resistant tomato tissues. J Phytopathol 148:181–190
Nakaho K, Inoue H, Takayama T, Miyagawa H (2004) Distribution and multiplication of Ralstonia solanacearum in tomato plants with resistance derived from different origins. J Gen Plant Pathol 70:115–119
Nakaho K, Kajihara H, Maeda M, Notsu A, Kawara T (2012) High-grafted tomatoes to control bacterial wilt caused by Ralstonia solanacearum (abstract). Phytopathology 102:S4.85
Schell MA (2000) Control of virulence and pathogenicity genes of Ralstonia solanacearum by an elaborate sensory network. Annu Rev Phytopathol 38:263–292
Schönfeld J, Heuer H, van Elsas JD, Smalla K (2003) Specific and sensitive detection of Ralstonia solanacearum in soil on the basis of PCR amplification of fliC fragments. Appl Environ Microbiol 69:7248–7256
Tanina K, Kawaguchi A (2012) Effect of different removal methods of diseased tomato plants of bacterial canker and bacterial wilt on transmission (abstract in Japanese). Jpn J Phytopathol 78:288
Turhan A, Ozmen N, Serbeci MS, Seniz S (2011) Effects of grafting on different rootstocks on tomato fruit yield and quality. Holt Sci 38:142–149
Wakimoto S (1955) Studies on the multiplication of OP1 phage (Xanthomonas oryzae bacteriophage) 1. One-step growth experiment under various conditions (in Japanese). Sci Bull Fac Agric Kyushu Univ 15:151–160
Acknowledgements
This work was supported by a Grant-in-Aid for the Science and Technology Research Promotion Program for Agriculture, Forestry, Fisheries and Food Industry from the Ministry of Agriculture, Forestry and Fisheries, Japan (25062C).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Inoue, Y., Kawaguchi, A. & Nakaho, K. Bacterial wilt-resistant tomato rootstock suppresses migration of ralstonia solanacearum into soil. J Gen Plant Pathol 84, 118–123 (2018). https://doi.org/10.1007/s10327-018-0771-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10327-018-0771-x