Abstract
Exposure of wildlife to anticoagulant rodenticides from sewer baiting and bait application is poorly understood. We analyzed residues of eight anticoagulant rodenticides in liver samples of 96 great cormorants, 29 common mergansers, various fish species, and coypu, in different German regions. Results show that hepatic residues of anticoagulant rodenticides were found in almost half of the investigated cormorants and mergansers due to the uptake of contaminated fish from effluent-receiving surface waters. By contrast, exposure of coypu to rodenticides via aquatic emissions was not observed. The maximum total hepatic anticoagulant rodenticide concentration measured in waterfowl specimens was 35 ng per g based on liver wet weight. Second-generation anticoagulant rodenticide active ingredients brodifacoum, difenacoum, and bromadiolone were detected almost exclusively, reflecting their estimated market share in Germany and their continuing release into the aquatic compartment. Overall, our findings reveal that second-generation anticoagulant rodenticides accumulating in wild fish are transferred to piscivorous predators via the aquatic food chain.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Exposure of wildlife to anticoagulant rodenticides via the terrestrial food web is a well-known and documented environmental issue (van den Brink et al. 2018). Less documented, however, are anticoagulant rodenticide emissions to the aquatic environment and the likely transfer of persistent, bioaccumulative, and toxic second-generation anticoagulants such as brodifacoum along the aquatic food chain (Regnery et al. 2019a, 2020). Two recent studies from Germany (Regnery et al. 2024) and Pennsylvania, North America (Facka et al. 2024) clearly reinforced the relevance of previously neglected aquatic exposure pathways (Lemarchand et al. 2014). Both studies frequently detected residues of anticoagulant rodenticides in primarily piscivorous mammalian predators, Eurasian otter (Lutra lutra) and river otter (Lontra canadensis), despite the nowadays strictly regulated sale, supply, and use of rodenticides (Facka et al. 2024; Regnery et al. 2024).
As transfer and fate of anticoagulant rodenticides in the aquatic food web are not yet fully disclosed, our biomonitoring study aimed at providing further experimental evidence concerning the exposure of piscivorous predators to second-generation anticoagulant rodenticides via their prey in densely inhabited landscapes, such as Germany. Hence, we analyzed liver samples of 125 specimens of two exclusively piscivorous avian predators, great cormorant (Phalacrocorax carbo) and common merganser (Mergus merganser), as well as 41 liver samples of various freshwater fish species from different German regions (Bavaria, Rhineland-Palatinate, Saxony, Lower Saxony) regarding residues of all eight active ingredients used in biocidal anticoagulant rodenticides in Germany. Moreover, liver samples of 42 specimens of a semi-aquatic living, mammalian herbivore (coypu (Myocastor coypus)) from Lower Saxony, a region with previously documented rodenticide burden in otters (Regnery et al. 2024), were analyzed to compare their risk of exposure versus that of piscivores. We hypothesized that exposure of aquatic top predators to anticoagulant rodenticides is diet-driven, and coypu, unlike cormorants and mergansers, are thus less likely to be exposed. Chemical analyses were accompanied by post-mortem examinations of cormorant and coypu carcasses.
Experimental
Piscivorous waterfowl
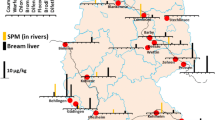
The randomly investigated 96 great cormorants (P. carbo) from southern (Bavaria, n = 50), western (Rhineland-Palatinate, n = 21), north-western (Lower Saxony, n = 1), and eastern (Saxony, n = 24) parts of Germany (Fig. 1) belonged to the continental subspecies P. carbo sinensis. All cormorants had been shot near surface waters for nature conservation reasons based on state-specific species protection exception regulations between 2020 and 2023 (outside breeding season) and their carcasses were provided for post-mortem examination. In Germany, P. carbo sinensis inhabits the coastal areas as well as inland surface waters, with breeding occurrences in suitable habitats. Outside breeding season, encountered individuals can be sedentary birds, partial migrants, or migratory birds, respectively, as the Baltic Sea population generally migrates overland and winters from southern Germany to North Africa. Due to their vast foraging grounds and high mobility (cormorants may roam widely during the day and visit multiple feeding waters), exact origins of their fish prey cannot be determined with certainty.
Twenty-nine liver tissue samples of common mergansers (M. merganser) were received from an on-going research project (FKZ A/20/03) about deterrence measures for nature conservation by Technical University of Munich, Wildlife Biology and Management Unit in collaboration with the Bavarian State Research Center for Agriculture, Institute for Fisheries. Adult birds had been culled at 6 selected stream sites in southern Germany (Fig. 1) in early spring 2023 (prior to the start of breeding season). In southern Bavaria, the common merganser lives as a sedentary bird year-round, with additional individuals passing through during winter months. Similar to cormorants, their prey consists primarily of small fish the size of 10–15 cm, which they hunt by diving in open surface waters. Thus, their foraging grounds generally overlap with those of great cormorants.
Freshwater fish
Freshwater fish sampling sites (Fig. 1) were in the broader vicinity of potential foraging grounds of analyzed cormorants and mergansers and included two streams each in Lower Saxony (Innerste, Leine) and Rhineland-Palatinate (Moselle, Queich), one stream in Saxony (Elbe), as well as one lake (Starnberger See) and three streams (Main, Isar, Pegnitz) in Bavaria. Individual (n = 35) and pooled (n = 6) liver tissue samples of species from different trophic levels such as common nase (Chondrostoma nasus), bleak (Alburnus alburnus), roach (Rutilus rutilus), chub (Squalius cephalus), brown trout (Salmo trutta f. fario), perch (Perca fluviatilis), pike (Esox lucius), pike-perch (Sander lucioperca), and European catfish (Silurus glanis) were kindly provided by the Bavarian Environment Agency, the Lower Saxony Water Management, Coastal and Nature Protection Agency, the Structural and Approval Directorate South (Upper Fisheries Authority) Rhineland-Palatinate, and the River Basin Community Elbe. The majority of liver tissue samples originated from fish caught between 2019 and 2023 during European Water Framework Directive biota monitoring campaigns.
Semi-aquatic living rodent
M. coypus, a semi-aquatic, invasive alien species with a plant-based diet, is classified as huntable game in most German federal states. A total of 42 coypu carcasses were obtained for post-mortem investigations from 17 different surface water locations in Lower Saxony (Fig. 1), at which coypu had been culled by hunters within the exercise of hunting rights between November 2020 and April 2021. Coypu are mainly nocturnal and crepuscular, respectively, and tend to stay along banksides during foraging.
Post-mortem investigation
Great cormorant carcasses from Saxony were examined according to routine procedures at the Museum of the Westlausitz Kamenz, whereas cormorant carcasses from Rhineland-Palatinate and Bavaria were handled at the Bavarian Environment Agency. Post-mortem examination of coypu carcasses and the single great cormorant from Lower Saxony was conducted at the Institute for Terrestrial and Aquatic Wildlife Research, University of Veterinary Medicine Hannover, Foundation. Recorded parameters for both species included biometric data, sex, estimated age, and nutrition status. For several specimens, the stomach content was also exemplarily recorded. Freezing of the carcasses prior to examination had prevented adequate blood sampling to screen for acute anticoagulant rodenticide poisoning characterized by coagulopathy. All sampled liver tissue was immediately frozen and shipped express on ice to the Federal Institute of Hydrology laboratory for chemical analyses.
Analytical methods and data analysis
Established analytical methods (Regnery et al. 2019b, 2024) were used for the quantitative chemical analysis of one pharmaceutical (phenprocoumon) and 8 biocidal (brodifacoum, bromadiolone, difenacoum, difethialone, flocoumafen, coumatetralyl, chlorophacinone, warfarin) anticoagulant active ingredients in liver tissue samples by liquid chromatography–tandem mass spectrometry. Method performance parameters for investigated species such as average recovery rates, method quantification limits, and estimated expanded measurement uncertainties are summarized in Tables S1–S3 (Supplementary Material) or already provided elsewhere (Regnery et al. 2019b, 2024). All reported analyte concentrations in liver tissue are based on wet weight. In addition, total hepatic lipid content of selected specimens was determined as described in Regnery et al. (2019b). Whenever total anticoagulant rodenticide concentrations are discussed in the following, residues of biocidal anticoagulants had been summed for each specimen, i.e., at least one of eight active ingredients detected above its respective method quantification limit, zero assigned for values below these limits. OriginPro, version 2021b (OriginLab Corporation, Northampton, MA, USA) was used for graphing and nonparametric Kruskal–Wallis analysis. Statistical difference was considered significant when p < 0.05.
Results and discussion
Age, sex, and body condition of examined specimens
The majority of investigated cormorants (i.e., 44 juveniles, 52 adults) was well nourished. Their determined total hepatic lipid contents were in the range of 2.7 ± 1.3% (in mergansers 5.0 ± 0.5%). The average body weights of female (n = 34) and male (n = 61) cormorants were 2182 ± 336 g and 2570 ± 321 g, respectively. Almost all cormorants had numerous nematodes in their gastrointestinal tracts. While stomach contents mainly consisted of small fish the size of 7–15 cm total length, a few larger fish up to 26 cm total length were also found. Identified ingested fish species were carp (Cyprinus carpio), chub, roach, and perch. The health condition of investigated coypu was predominantly good. Approximately two thirds were well nourished and observed stomach contents were considered typical for this herbivorous species. The average body weight of investigated coypu (i.e., 16 juveniles, 26 adults) was 3732 ± 1591 g for females (n = 18) and 4651 ± 1798 g for males (n = 23). Determined total hepatic lipid contents were in the range of 3.2 ± 0.6%.
Measured hepatic second-generation anticoagulant rodenticide residues
Overall, 46 out of 96 cormorants (47.9%) from all four regions exhibited quantifiable anticoagulant rodenticide residues in their livers, mostly from 1–2 second-generation anticoagulant rodenticide active ingredients with a maximum total anticoagulant rodenticide burden of 35.1 ng/g (Fig. 2). Concentrations measured in males and females indicated no statistical difference (Kruskal–Wallis test, H(1) = 0.342, p = 0.559). Brodifacoum was detected in 39 (max. concentration of 27.6 ng/g), difenacoum in 23 (max. 7.5 ng/g), and bromadiolone in 3 (max. 2.3 ng/g) specimens, respectively. Coumatetralyl was solely detected in one cormorant liver tissue sample at very low concentration (0.18 ng/g), corroborating the lesser bioaccumulation potential of first-generation anticoagulant rodenticides. In good agreement with findings from cormorants shot near Bavarian surface waters (Fig. 2), hepatic anticoagulant rodenticide residues were also detected in 13 out of 29 mergansers (44.8%), mostly from one second-generation active ingredient. Brodifacoum was detected in 12 specimens (max. concentration of 9.4 ng/g), bromadiolone in 2 (max. 1.6 ng/g), and difenacoum in one (0.5 ng/g), respectively. Residue levels of brodifacoum, difenacoum, and bromadiolone were not related to hepatic total lipid contents. Flocoumafen, difethialone, chlorophacinone, warfarin, and the pharmaceutical anticoagulant phenprocoumon were not detected above their respective method quantification limits in the analyzed waterfowl liver samples.
Box plots of measured total anticoagulant rodenticide residue concentrations in liver tissue samples of investigated cormorants and mergansers from different German regions that had been shot near surface waters between 2020 and 2023. Residues of detected biocidal anticoagulants had been summed for each specimen, zero was assigned for values below the respective method quantification limits. Overall, 46 out of 96 cormorants (47.9%) and 13 out of 29 mergansers (44.8%) exhibited quantifiable anticoagulant rodenticide residues in their livers, mostly from 1 to 2 second-generation anticoagulant rodenticide active ingredients with a maximum total anticoagulant rodenticide burden of 35.1 ng/g based on wet weight. Rodenticide residue concentrations were not significantly different among groups, i.e., among all cormorants and cormorants and mergansers from Bavaria (Kruskal–Wallis test, H(2) = 0.773, p = 0.679)
In contrast, solely one adult coypu exhibited elevated residues of 135.4 ng/g difenacoum in its liver, together with traces of a second active ingredient (1.1 ng/g brodifacoum). It should be emphasized that none of the biocidal and pharmaceutical anticoagulants were detected in any of the other 41 analyzed coypu. Thereof were 3 specimens that had been culled at the same location as the exposed one. In wild freshwater fish, measured total hepatic anticoagulant rodenticide concentrations (Fig. 3) matched previous records of rodenticides in fish from these effluent-receiving streams, e.g., Main, Isar (Regnery et al. 2019b), Elbe (Kotthoff et al. 2019), Moselle, Queich (Regnery et al. 2020), illustrating the continued emission of rodenticides from sewer baiting and outdoor surface baiting into the aquatic compartment. Their absence in fish from Starnberger See, an effluent-free lake, was also in good agreement with previous records (Regnery et al. 2019b). Highest total hepatic second-generation anticoagulant rodenticide levels in fish (mainly brodifacoum) of 74.5 ng/g (roach, 26 cm total length) and 95.6 ng/g (chub, 30.5 cm total length) were detected at two stream sites in Rhineland-Palatinate (Queich) and Lower Saxony (Innerste), respectively. At both sites, sewer baiting measures using baits deployed by wire in combined sewer systems had been carried out shortly before fish sampling campaigns, according to released public press communications.
Mean total anticoagulant rodenticide residue concentrations in liver tissue samples (n = 41) of different herbivorous (hv), omnivorous (ov), and inverti-/piscivorous (iv/pv) fish species from multiple surface water sampling sites located in Bavaria (B), Rhineland-Palatinate (RP), Lower Saxony (LS), and Saxony (S). Concentrations of detected biocidal anticoagulants, based on liver wet weight, had been summed for each specimen. Specimens were grouped by feeding-type, which presumably is a determining factor in second-generation anticoagulant rodenticide uptake. Where applicable, the relative standard deviation of mean values is shown. Highest total hepatic second-generation anticoagulant rodenticide levels in fish were observed at two stream sites (Queich, Innerste) with nearby sewer baiting
Diet-driven exposure risk
As mentioned earlier, the exact origins of the waterfowl’s ingested fish prey, and thus second-generation rodenticide residues, were unknown. Four cormorant individuals shot at surface waters in Bavaria had been tagged in Latvia, Finland, Switzerland, and Northern Germany, respectively. The limited and unforeseeable availability of biological tissue samples from protected species did not allow for strategic collection of corresponding predator and prey samples to ascertain full spatial and temporal overlap. Moreover, the prey composition of cormorants usually depends on what fish can be caught at all, or with as little effort as possible, rather than a strong preference for certain fish species (Keller 1998). Yet, the continuous presence of hepatic second-generation anticoagulant rodenticides in fish from effluent-receiving streams in the vicinity of foraging grounds of analyzed cormorants and mergansers demonstrates that exposure of piscivorous avian predators occurs via their fish prey. Residue levels in the analyzed waterfowl also clearly reflected current use patterns and the market dominance of brodifacoum, difenacoum, and bromadiolone containing biocidal products in Germany (Regnery et al. 2024). Another unequivocal indication was the absence of low-level anticoagulant rodenticide residues in coypu from Lower Saxony, a region previously known for pronounced anticoagulant rodenticide use and thus frequent detection in otters (Regnery et al. 2024). As pointed out in a recent review, including species from a diversity of trophic levels during biomonitoring is very helpful to comprehend exposure pathways (Keating et al. 2024). Primary exposure to difenacoum-containing bait was deemed most plausible to explain the elevated concentration detected in one adult coypu. Although their body size should prevent them from directly accessing tamper-resistant bait station, loose grain bait may be attractive for coypu when accessible. For instance, when baits are spilled from bait stations deployed near banks or deliberately offered.
Primary exposure of cormorants and mergansers to rodenticide bait, on the other hand, is considered extremely unlikely. The seemingly low hepatic rodenticide levels of investigated piscivorous waterfowl (Fig. 2) compared to reported secondary poisoning levels in predatory wildlife of the terrestrial food web (van den Brink et al. 2018) can most likely be explained by the absence of residues in fish from fish rearing ponds and surface waters without wastewater-borne rodenticide emissions (Regnery et al. 2019b; Kotthoff et al. 2019) that are frequently visited by cormorants during foraging (Keller 1998). Additional factors concerning piscivorous avian predators, such as the regurgitation of food if alarmed and a higher body temperature compared to mammals, may play a role too in terms of bioaccumulation and biotransformation (Kuo et al. 2022). The absence of second-generation anticoagulant rodenticides in 5 liver samples of common nase, a predominantly herbivorous fish species, also suggests that the foraging strategy is a determining factor in second-generation anticoagulant rodenticide uptake in the aquatic food web, e.g., such as the diversity and complexity of diets. Other fish caught at the same time at the Isar sampling site exhibited hepatic rodenticide residues in comparison (Fig. 3). However, more research (and data) will be required for a sound statistical assessment of such complex food web relationships.
Conclusion
Extensive knowledge and understanding of actual exposure pathways of biocidal anticoagulant rodenticides is essential to improve environmental exposure and risk assessments, and consequentially risk mitigation measures for the aquatic environment. Our biomonitoring study demonstrated that piscivorous avian predators in anthropogenically influenced landscapes are exposed to second-generation anticoagulant rodenticides via their fish prey. Transfer of second-generation active ingredients along the aquatic food chain was thus confirmed. Without doubt, future improvements of regulatory measures concerning biocides will be required to mitigate the yet unknown consequences for aquatic wildlife from the nowadays almost exclusive application of second-generation anticoagulant rodenticides during chemical rodent control.
Data availability
Data will be made available on request.
Code availability
Not applicable.
References
Facka A, Frair J, Keller T, Miller E, Murphy L, Ellis JC (2024) Spatial patterns of anticoagulant rodenticides in three species of medium-sized carnivores in Pennsylvania. Can J Zool 00:1–12. https://doi.org/10.1139/cjz-2023-0131
Keller T (1998) Die Nahrung von Kormoranen (Phalacrocorax carbo sinensis) in Bayern. J Ornithol 139:389–400. https://doi.org/10.1007/BF01653465
Keating MP, Saldo EA, Frair JL, Cunningham SA, Mateo R, Jachowski DS (2024) Global review of anticoagulant rodenticide exposure in wild mammalian carnivores. Anim Conserv. https://doi.org/10.1111/acv.12947
Kotthoff M, Rüdel H, Jürling H et al (2019) First evidence of anticoagulant rodenticides in fish and suspended particulate matter: spatial and temporal distribution in German freshwater aquatic systems. Environ Sci Pollut Res Int 26:7315–7325. https://doi.org/10.1007/s11356-018-1385-8
Kuo DTF, Rattner BA, Marteinson SC et al (2022) A critical review of bioaccumulation and biotransformation of organic chemicals in birds. Rev Environ Contam Toxicol 260:6. https://doi.org/10.1007/s44169-021-00007-1
Lemarchand C, Rosoux R, Talon C, Berny P (2014) Flagship species conservation and introduced species invasion: toxic aspects along Loire River (France). In: Larramendy ML, Soloneski S (eds) Pesticides—toxic aspects. InTech, Rijeka, pp 53–79
Regnery J, Friesen A, Geduhn A et al (2019a) Rating the risks of anticoagulant rodenticides in the aquatic environment: a review. Environ Chem Lett 17:215–240. https://doi.org/10.1007/s10311-018-0788-6
Regnery J, Parrhysius P, Schulz RS et al (2019b) Wastewater-borne exposure of limnic fish to anticoagulant rodenticides. Water Res 167:115090. https://doi.org/10.1016/j.watres.2019.115090
Regnery J, Schulz RS, Parrhysius P et al (2020) Heavy rainfall provokes anticoagulant rodenticides’ release from baited sewer systems and outdoor surfaces into receiving streams. Sci Total Environ 740:139905. https://doi.org/10.1016/j.scitotenv.2020.139905
Regnery J, Rohner S, Bachtin J et al (2024) First evidence of widespread anticoagulant rodenticide exposure of the Eurasian otter (Lutra lutra) in Germany. Sci Total Environ 907:167938. https://doi.org/10.1016/j.scitotenv.2023.167938
van den Brink NW, Elliott JE, Shore RF, Rattner BA (eds) (2018) Anticoagulant rodenticides and wildlife. Springer Nature, Cham
Acknowledgements
We are very grateful to Manfred Fetthauer and colleagues at ARGE Nister/Obere Wied e.V., Matthias Ruff and colleagues at the Bavarian Environment Agency, and Marcello Petrone at the Upper Fisheries Authority, Rhineland-Palatinate for providing cormorant carcasses and fish tissue samples, respectively.
Funding
Support of this study was provided by the German Environment Agency through Grant FKZ 3720 64 409 0.
Author information
Authors and Affiliations
Contributions
Julia Regnery: Conceptualization, Validation, Formal analysis, Investigation, Writing—original draft, Funding acquisition; Hannah Schmieg: Data curation, Investigation, Writing—review & editing; Hannah Schrader: Data curation, Investigation, Writing—review & editing; Olaf Zinke: Data curation, Writing—review & editing; Friederike Gethöffer: Data curation, Investigation, Writing—review & editing; Sarah-Alica Dahl: Data curation, Writing—review & editing; Mario Schaffer: Data curation, Writing—review & editing; Julia Bachtin: Investigation, Writing—review & editing; Christel Möhlenkamp: Investigation, Writing—review & editing; Anton Friesen: Conceptualization, Writing—review & editing, Supervision.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Ethical approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Regnery, J., Schmieg, H., Schrader, H. et al. Rodenticide contamination of cormorants and mergansers feeding on wild fish. Environ Chem Lett (2024). https://doi.org/10.1007/s10311-024-01762-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10311-024-01762-y