Abstract
Classical purification of pharmaceuticals is energy-intensive and employs toxic solvents that are discarded, calling for more sustainable methods. Here, we purified tetracycline by organic solvent forward osmosis using ionic liquids. Results show the osmotic enrichment of feed solutions containing different concentrations of tetracycline in methanol. The solvent flux during the filtration process is mainly influenced by solvent properties, such as molecular size, viscosity, polarity, and the solvent–membrane interaction. We evaporated the diluted draw solution to recover the draw solute for reuse. Overall ionic liquids appear as suitable draw solutes for organic solvent forward osmosis for pharmaceutical compound enrichment with draw solute recovery and reuse.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
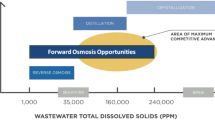
During drug synthesis, active pharmaceutical ingredients are typically dissolved in organic solvents (Rantanen and Khinast 2015). Thus, the separation and purification of pharmaceutical compounds in organic solvents are necessary in improving the final product quality. These account for over half of the equipment investment and operating costs in pharmaceutical industry (Wang et al. 2021). Active pharmaceutical separation has specific requirements because (1) pharmaceutical compounds are mostly temperature-sensitive, and high temperatures may cause pharmaceutical decomposition (Geens et al. 2007), and (2) the process should not pollute the solvent stream due to manufacturing process sensitivity (Grodowska and Parczewski 2010). Considering these factors, membrane technology has attracted attention due to its low energy consumption and phase change elimination (Gonzales et al. 2022a). Organic solvent nanofiltration is commonly used in the pharmaceutical industry for organic solvent applications, such as organic solvent recovery and pharmaceutical enrichment (Kato et al. 2021). However, this process has limitations, since applied pressure may result in additional operating costs and membrane fouling.
Forward osmosis is an osmotically driven membrane process that utilizes the salinity difference between the draw and feed solutions (Kaleekkal et al. 2022). There is spontaneous solvent transfer from the feed to draw solutions across a semi-permeable membrane, effectively enriching the feed and diluting the draw. Recently, organic solvent forward osmosis has emerged as an alternative separation process in the pharmaceutical industry (Zhou et al. 2020; Wei et al. 2021). This process has demonstrated feasibility in concentrating pharmaceutical products at the feed side while simultaneously delivering organic solvent to the draw side (Lively and Sholl 2017). The diluted draw solution can be regenerated through filtration, distillation, and evaporation (Cui and Chung 2018). Compared to organic solvent nanofiltration, organic solvent forward osmosis has reduced fouling tendency since no pressure is applied. Additionally, by choosing a suitable draw solute, sufficient osmotic pressure can be generated, which can be challenging to achieve in pressure-driven membrane processes due to high operating pressure requirements (Lively and Sholl 2017). Despite these advantages, organic solvent forward osmosis is still an emerging field, and several issues need to be addressed. For instance, reverse draw solute diffusion and concentration polarization are factors that compromise the osmotic performance (Zhao and Zou 2011).
One major challenge in forward osmosis is the limited suitable regenerable draw solutes available (Ray et al. 2018). Several compounds have previously been proposed as draw solutes (Zhong et al. 2016); however, regeneration can be energy-intensive and toxic (Ge et al. 2013). Similarly, organic solvent forward osmosis requires suitable draw solutes that exhibit low or no reverse diffusion and ease in regeneration. In earlier work, LiCl was used as draw solute (Cui and Chung 2018); however, reverse LiCl diffusion reduces the driving force and increases potential hazards (Ma et al. 2022). Additionally, LiCl has limited solubility in organic solvents (Avdibegović et al. 2022); thus, a higher-concentrated LiCl solution could precipitate during operation and regeneration by solvent evaporation. Recently, ionic liquids, composed of large organic ions and can have low melting points below 100 °C at atmospheric pressure (Yi et al. 2015), have been explored as draw solutes (Kamio et al. 2021) and found to be easily separated from solution by simple heating due to their non-volatility and recoverability (Rajadurai and Anguraj 2021).
This study introduces the ionic liquid-drawn organic solvent forward osmosis process for pharmaceutical enrichment. Polyketone-supported thin-film composite membrane was employed in this investigation for its organic solvent resistance (Kushida et al. 2022). Osmotic performance was systematically investigated by utilizing different solvents and draw solutes. Tetracycline, a veterinary and agricultural antibiotic (Daghrir and Drogui 2013), was chosen as the model pharmaceutical compound due to the high rejection of highly crosslinked membranes against tetracycline. Additionally, tetracycline can be readily detected and measured through spectrophotometry. The draw solute was recovered by evaporation and recycled to maintain the draw solution concentration. To the authors’ best knowledge, this is the first study on continuous ionic liquid-drawn organic solvent forward osmosis and regeneration.
Experimental
Membrane fabrication and membrane performance evaluation
The polyketone-supported thin film composite membrane was fabricated according to our earlier work (Gonzales et al. 2021; Li et al. 2022), described in the Supplementary Material. The osmotic performance of the membrane was evaluated using an organic solvent-resistant forward osmosis system (Figure S1) (Gonzales et al. 2022b). Methanol, ethanol, and 2-propanol, whose properties are given in Table S1, were selected as the organic solvents. LiCl, polyethylene glycol 400, 1-butyl-3-methylimidazolium tetrafluoroborate, 1-butyl-3-methylimidazolium bis(trifluoromethylsulfonyl)imide, and 1-ethyl-3-methylimidazolium bis(trifluoromethylsulfonyl)imide were employed as draw solutes. Their molecular weights and chemical structures are provided in Table S2. Intrinsic transport properties were determined (Guan et al. 2021) and are presented in Table S3. All experimental details, including viscosity, conductivity, osmotic pressure (Figure S2), and solute concentration (Figure S3) measurements, are shown in the Supplementary Material.
Organic solvent forward osmosis operation for pharmaceutical enrichment
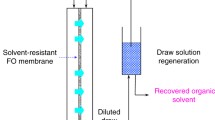
The enrichment of tetracycline was demonstrated through a continuous system comprising two parts (Fig. 1), forward osmosis and draw solute recovery systems. After a predetermined operational period, the operation was halted, and the diluted draw solution was collected. Subsequently, the solvent and draw solute were separated using a vacuum rotary evaporator and reused for re-operation. Detailed information can be found in the Supplementary Material.
Results and discussion
Choosing the suitable solvent and draw solute system
The osmotic performance was assessed using various alcoholic solvents and draw solutes to determine the optimal solvent–draw solute system. Methanol exhibited the highest flux, followed by ethanol and 2-propanol. The results can be attributed to the solvent properties, including molecular size, viscosity, and polarity, as well as the interaction between solvent molecules and the membrane, as detailed in Supplementary Material. The smaller molecular size of methanol compared to ethanol and 2-propanol mitigated mass transfer resistance, resulting in higher flux. Also, higher viscosity can exacerbate the occurrence of concentration polarization and effectively reduce the effective osmotic driving force. Lastly, the polarity of the solvent generated a higher degree of dissociation of the draw solute; thus, the methanol solution exhibited the highest osmotic pressure compared to the ethanol and 2-propanol solutions. Also, due to the polar nature of the polyamide-based membrane, methanol, whose polarity was the highest among the solvents, could exhibit strong electrostatic interactions with the membrane, resulting in higher solvent flux.
Aside from LiCl, various types of draw solutes, including ionic liquids and polyethylene glycol 400, were investigated to identify the most suitable solute that offers relatively high solvent flux, low reverse draw salt flux, and applicability for pharmaceutical enrichment. Table 1 presents the solution properties (viscosity and osmotic pressure), as well as the methanol flux and reverse draw solute flux. Despite the highest osmotic pressure generated by the polyethylene glycol 400 solution, the lowest flux among all systems was observed, mainly due to the solution viscosity and its influence on concentration polarization occurrence. The ionic liquid draw solutes, on the other hand, generated comparably high solvent flux, and no reverse draw solute flux was detected for all systems, indicating excellent membrane selectivity against ionic liquids, in comparison with LiCl. Also, ionic liquids exhibit other benefits, such as applicability to pharmaceutical enrichment and draw solute regeneration through simple evaporation due to their nonvolatile nature. Due to the highest osmotic pressure generation and solvent drawing capability of the 2.0M 1-ethyl-3-methylimidazolium bis(trifluoromethylsulfonyl)imide solution, this was chosen as the optimal draw solute. In the subsequent work, the draw solute was recovered and recycled during a continuous process.
Organic solvent forward osmosis operation for pharmaceutical enrichment
Pharmaceutical enrichment via organic solvent forward osmosis was demonstrated using different concentrations of tetracycline (20, 200, and 2000 mg/L) in methanol and 2.0 M 1-ethyl-3-methylimidazolium bis(trifluoromethylsulfonyl)imide in methanol as feed and draw solutions, respectively. Tetracycline retention using the thin-film composite membrane was predetermined to be 95.7%. During the osmotic process, methanol transport from the feed to draw side would enrich the feed solution and dilute the draw solution, and the osmotic pressure difference across the membrane would become lower, resulting in solvent flux decline. Using the low boiling point of methanol and nonvolatility of 1-ethyl-3-methylimidazolium bis(trifluoromethylsulfonyl)imide, it is possible to separate it from methanol by a simple evaporation and recycle the draw solute to minimize the decreased solvent flux during a continuous operation. While evaporation may be considered energy-intensive, the low boiling point of methanol allows for the utilization of low-quality energy sources such as waste heat, making it an easily achievable process.
As depicted in Fig. 2, the flux exhibited a decline during the osmotic operation using all concentrations of tetracycline as feed. This decrease was primarily attributed to the dilution of the draw solution. By separating the components of the diluted draw solution and ionic liquid recovery, it was possible to reconstitute a 2.0 M solution, resulting in the solvent flux recovery for all experiments (Figs. 2a-1–a-3). Figures 2b-1–2b-3 illustrate the change in feed solution volume versus the course of operation. At the end of the operation, the volumes of the feed solutions had decreased to approximately one-third of the initial volumes. The final tetracycline concentrations were measured to be 58.0, 566, and 5450 mg/L for the experiments utilizing initial feed solutions of 20, 200, and 2000 mg/L tetracycline, respectively. The concentration factor was calculated to be around 3 for all cases, indicating the successful enrichment of the tetracycline-containing feed solution through organic solvent forward osmosis operation, irrespective of the initial tetracycline concentration in the feed. After 1-ethyl-3-methylimidazolium bis(trifluoromethylsulfonyl)imide recovery, no changes in the chemical structure of the ionic liquid (Figure S10) were observed, confirming the feasibility of ionic liquid recycling and reuse within this process. In real-world applications of this process, coupling of forward osmosis and evaporation is recommended, enabling complete continuous operation with feed solution enrichment and draw solute recovery.
Time course of a solvent flux and b normalized feed volume during organic solvent forward osmosis operation for tetracycline enrichment. Feed solution: (1) 20 mg/L (volume: 1.0 L, operation time: 2500 min), (2) 200 mg/L (volume: 0.5 L, operation time: 700 min), (3) 2000 mg/L (volume: 0.1 L, operation time: 660 min) tetracycline in methanol, Draw solution: 2.0 M 1-ethyl-3-methylimidazolium bis(trifluoromethylsulfonyl)imide in methanol, Flow rate: 300 mL/min, Orientation: active-layer-facing-feed-solution. Draw solution recycling was conducted during long-term operation. Successful tetracycline enrichment was observed regardless of initial tetracycline concentration
Conclusion
This work assessed the feasibility of ionic liquid-drawn organic solvent forward osmosis operation for pharmaceutical enrichment. The selection of appropriate solvents and solutes was investigated, and the first operation of continuous organic solvent forward osmosis with draw solute recovery was demonstrated. The performance of solvent flux was influenced by the solvent molecular size, viscosity, polarity, and the interaction between solvent molecules and the membranes. Using ionic liquid, 1-ethyl-3-methylimidazolium bis(trifluoromethylsulfonyl)imide, as draw solute, demonstrated high solvent flux, negligible reverse draw solute flux, and regenerability. Successful pharmaceutical compound enrichment was achieved, and the ionic liquid was effectively recovered and reused. These findings highlight the potential of ionic liquid-drawn organic solvent forward osmosis for continuous pharmaceutical enrichment, accompanied by draw solute recovery.
Data availability
Data and material are available upon request.
References
Avdibegović D, Nguyen VT, Binnemans K (2022) One-step solvometallurgical process for purification of lithium chloride to battery grade. J Sustain Metall 8:893–899
Cui Y, Chung TS (2018) Pharmaceutical concentration using organic solvent forward osmosis for solvent recovery. Nat Commun 9:1426
Daghrir R, Drogui P (2013) Tetracycline antibiotics in the environment: a review. Environ Chem Lett 11:209–227
Ge Q, Ling M, Chung TS (2013) Draw solutions for forward osmosis processes: developments, challenges, and prospects for the future. J Membr Sci 442:225–237
Geens J, De Witte B, Van der Bruggen B (2007) Removal of API’s (active pharmaceutical ingredients) from organic solvents by nanofiltration. Sep Sci Technol 42:2435–2449
Gonzales RR, Zhang L, Sasaki Y, Kushida W, Matsuyama H, Shon HK (2021) Facile development of comprehensively fouling-resistant reduced polyketone-based thin film composite forward osmosis membrane for treatment of oily wastewater. J Membr Sci 626:119185
Gonzales RR, Kato N, Awaji H, Matsuyama H (2022a) Development of polydimethylsiloxane composite membrane for organic solvent separation. Sep Purif Technol 285:120369
Gonzales RR, Sasaki Y, Istirokhatun T, Li J, Matsuyama H (2022b) Ammonium enrichment and recovery from synthetic and real industrial wastewater by amine-modified thin film composite forward osmosis membranes. Sep Purif Technol 297:121534
Grodowska K, Parczewski A (2010) Organic solvents in the pharmaceutical industry. Acta Pol Pharm 67:1
Guan K, Sasaki Y, Jia Y, Gonzales RR, Zhang P, Lin Y, Li Z, Matsuyama H (2021) Interfacial polymerization of thin film selective membrane layers: effect of polyketone substrates. J Membr Sci 640:119801
Kaleekkal NJ, Nambikkattu J, Rasheeda Satheesh A, Gonzales RR, Shon HK, Vigneswaran S (2022) Engineered osmosis—sustainable technology for water recovery, product concentration and energy generation. Environ Sci Water Res Technol 8:1326–1358
Kamio E, Kurisu H, Takahashi T, Matsuoka A, Yoshioka T, Nakagawa K, Matsuyama H (2021) Using reverse osmosis membrane at high temperature for water recovery and regeneration from thermo-responsive ionic liquid-based draw solution for efficient forward osmosis. Membranes 11:588
Kato N, Gonzales RR, Nishitani A, Negi Y, Ono T, Matsuyama H (2021) Single-step preparation of nanocomposite polyamide 6 hollow fiber membrane with integrally skinned asymmetric structure for organic solvent nanofiltration. Colloids Surf A Physicochem Eng Asp 620:126538
Kushida W, Gonzales RR, Shintani T, Matsuoka A, Nakagawa K, Yoshioka T, Matsuyama H (2022) Organic solvent mixture separation using fluorine-incorporated thin film composite reverse osmosis membrane. J Mater Chem A 10:4146–4156
Li J, Gonzales RR, Takagi R, Yao X, Zhang P, Istirokhatun T, Zhang J, Matsuyama H (2022) Surface modification of thin film composite forward osmosis membrane using tris(2-aminoethyl)amine for enhanced ammonium recovery. Desalination 541:116002
Lively RP, Sholl DS (2017) From water to organics in membrane separations. Nat Mater 16:276–279
Ma L, Han X, Zhang S, Wang L (2022) Sulfonated metal–organic framework nanostructure-based membranes with precise sieving for organic solvent forward osmosis. ACS Appl Nano Mater 5:11324–11333
Rajadurai V, Anguraj BL (2021) Ionic liquids to remove toxic metal pollution. Environ Chem Lett 19:1173–1203
Rantanen J, Khinast J (2015) The future of pharmaceutical manufacturing sciences. J Pharm Sci 104:3612–3638
Ray SS, Chen S-S, Sangeetha D, Chang HM, Thanh CND, Le QH, Ku HM (2018) Developments in forward osmosis and membrane distillation for desalination of waters. Environ Chem Lett 16:1247–1265
Wang ZY, Feng R, Wang WJ, Sun YX, Tao SN, Wang YM, Chen YF, Fu ZJ, Cao XL, Sun SP, Xing W (2021) Robust braid reinforced hollow fiber membranes for organic solvent nanofiltration (OSN). Adv Membr 1:100007
Wei Y, Wang Y, Wang L, Yang H, Jin H, Lu P, Li Y (2021) Simultaneous phase-inversion and crosslinking in organic coagulation bath to prepare organic solvent forward osmosis membranes. J Membr Sci 620:118829
Yi Y-B, Lee JW, Chung C-H (2015) Conversion of plant materials into hydroxymethylfurfural using ionic liquids. Environ Chem Lett 13:173–190
Zhao S, Zou L (2011) Relating solution physicochemical properties to internal concentration polarization in forward osmosis. J Membr Sci 379:459–467
Zhong Y, Feng X, Chen W, Wang X, Huang KW, Gnanou Y, Lai Z (2016) Using UCST ionic liquid as a draw solute in forward osmosis to treat high-salinity water. Environ Sci Technol 50:1039–1045
Zhou Z, Hu Y, Wang Q, Mi B (2020) Carbon nanotube-supported polyamide membrane with minimized internal concentration polarization for both aqueous and organic solvent forward osmosis process. J Membr Sci 611:118273
Funding
Open access funding provided by Kobe University.
Author information
Authors and Affiliations
Contributions
All authors contributed to study conception and design; JL, RRG, YCC, AM did material preparation, data collection, and analysis; JL and RRG done writing; RRG, RT, LD, and HM reviewed and edited the study. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Li, J., Gonzales, R.R., Takagi, R. et al. Continuous purification of drugs by ionic liquid-drawn organic solvent forward osmosis and solute recovery. Environ Chem Lett 22, 29–34 (2024). https://doi.org/10.1007/s10311-023-01641-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10311-023-01641-y