Abstract
Polycyclic aromatic hydrocarbons are toxic pollutants which persist in the environment. Extraction of polycyclic aromatic hydrocarbons requires large volumes of toxic organic solvents, but the use of non-hazardous solvents provides a potentially cleaner approach to quantifying polycyclic aromatic hydrocarbons in environmental matrices. Here we investigated the efficiency of eucalyptus oil to extract polycyclic aromatic hydrocarbons from spiked soil and sediment. Eucalyptus oil extraction conditions including temperature, time, and volume of oil were optimized employing a Box–Behnken experimental design, and the desorption kinetics of phenanthrene, pyrene, chrysene, and benzo[a]pyrene were evaluated using an empirical first order kinetic model. Results show that extraction efficiency from soil, of 112% for phenanthrene, 108% for pyrene, 102% for chrysene and 98% for benzo[a]pyrene, is higher than that from sediment, of 90, 111, 84 and 82%, respectively. This may be due to soil possessing 12 times more organic carbon. Recoveries higher than 77% were obtained over the concentration range 0.5–10 mg/kg for the tested polycyclic aromatic hydrocarbons, with limits of detection lower than 63 µg/kg and limits of quantitation lower than 125 µg/kg. These findings suggest that eucalyptus oil has potential as a safer solvent to extract hydrophobic contaminants.
Similar content being viewed by others
Introduction
Polycyclic aromatic hydrocarbons (PAHs) are widespread environmental pollutants with two or more fused aromatic rings, which can accumulate in the soil, sediment, water, plants and animals, with 90% of global PAH burden present in soils and sediments (Wild et al. 1995). PAHs can be produced in high temperature, low oxygen conditions such as forest fires, combustion engines and during the production of coke and tar (Abdel-Shafy et al. 2016). More volatile low molecular weight PAHs (2–3 ringed) are present mainly in the vapour phase and high molecular weight PAHs (> 4 rings) mostly associate with the particulate phase in the environment (Abbas et al. 2018). PAHs and their derivatives may exhibit teratogenic, mutagenic, and carcinogenic effects to humans and other organisms (Abbas et al. 2018; Parales et al. 2002; Yu et al. 2013), making remediation of contaminated sites essential to allow land reuse.
Monitoring PAHs in the environment is essential to assessing PAH concentration and type, and for investigating the effectiveness of contaminated site bioremediation, but their extraction from solid matrices is inefficient due to the strong PAH-matrix interactions (Saldana et al. 2005). Soxhlet, ultrasonic, liquid–liquid and solid-phase extraction are commonly used methods, however, these techniques typically require large volumes of industrial organic solvents, which increases time, cost, waste, and poses a potential environmental hazard (Kariyawasam et al. 2022). Other less common techniques, such as Pasteur pipette extraction, have also been reported, but are limited to small sample sizes which may not be representative of samples with poor homogeneity (Henner et al. 1997). To mitigate these issues, vegetable oils have been used to extract PAHs from solid matrices due to their non-toxic and biodegradable nature, while improving the extraction efficiency and reducing the cost (Gong et al. 2006; Lau et al. 2012; Pannu et al. 2004; Yap et al. 2010).
The eucalyptus tree produces an essential oil, which can be used as an eco-friendly solvent and has been used in the soap industry due to its surface-active properties (Small 1981). The major component in eucalyptus oil is eucalyptol, with a logarithm of the octanol/water partition coefficient (log Kow) of 2.74 (PubChem 2022b), which gives it a similar polarity to toluene (log Kow = 2.73, PubChem (2022a)) and a slightly higher polarity than hexane (log Kow = 3.90, PubChem (2022c)). These physical properties may make eucalyptus essential oil a potentially cleaner solvent for PAH extraction, at a lower cost due to its low technology approach. The present study evaluates the efficacy of eucalyptus oil to extract PAHs from contaminated soil and sediment for the purpose of PAH quantitation, which has not been investigated to date. Further, oil extraction of PAHs for one type of oil has not been conducted for different matrices.
Experimental
Chemical standards
Stock solutions of phenanthrene, pyrene, chrysene and benzo[a]pyrene were prepared in acetonitrile (5 μg/mL), and deuterated PAHs, phenanthrene-D10, pyrene-D10, chrysene-D10 and benzo[a]pyrene-D12 were used as internal standards (10 μg/mL).
Spiking of soil and sediment
Two grams of soil or sediment (Text S1 and Table S1) were weighed into glass screw cap vials in triplicate and covered with acetone (1800 µL), (Guo et al. 2013) and were spiked with PAHs (800 µL, 5 μg/mL). Acetone was evaporated under a gentle stream of nitrogen resulting in PAH concentrations in soil and sediment of 2 mg/kg.
Eucalyptus oil extraction of polycyclic aromatic hydrocarbons
Eucalyptus oil (4–6 mL), confirmed to be PAH free by gas chromatography-mass spectrometry (GC–MS), was added to PAH spiked soil samples (2.00 g), and the soil slurries were mechanically mixed by inversion for 3–7 days at 15–25 °C. The extracts were centrifuged (3220 × g, 5 min), and the supernatants were spiked with the internal standards (50 µL, 10 μg/mL deuterated PAHs), to account for the losses during the filtration and evaporation steps, and minor variations in resuspension volume. The filtered extracts were analysed by GC–MS, (Text S2). A minimum of 4 mL was required to minimise deviations in the volume of solvent recovered after centrifugation.
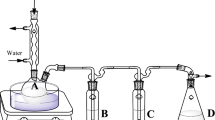
A second-order multivariate Box–Behnken experimental design was employed to optimize extraction parameters, such as temperature, solvent volume, and extraction time, and the experimental design is shown in supplementary Text S3 and Table S2, and levels were selected based on studies conducted for vegetable oils (Gong et al. 2005a; Pannu et al. 2004). However, a lower temperature range was selected in this study due to the lower flash point of eucalyptus oil compared to commonly used vegetable oils (Devan et al. 2009). Steps followed during oil extraction of PAHs from soil/sediment and the optimization have been summarized in Fig. 1.
Experimental procedure of the eucalyptus oil extraction of polycyclic aromatic hydrocarbons (PAHs). X denotes shaking period and T denotes temperature. Extraction conditions including temperature, time and solvent volume were varied to obtain the optimum conditions. PAH extracts obtained after centrifugation were filtered and analysed using gas chromatography-mass spectrometry (GC–MS). Response surface methodology was applied to optimize the process parameters
Method validation
Under the most favourable oil extraction conditions, the limits of detection, limits of quantification, linearity and recovery efficiencies (in the range of 0.5–10 mg/kg) were used to validate the proposed method. Limits of detection were taken as the lowest concentration of a PAH that could be detected through visual evaluation and relative standard deviation < 10% was considered as the limit of quantification. Statistical analysis was conducted using one-way analysis of variance and Tukey's posthoc test (Wawra et al. 2018). A two‑tailed P < 0.05 was considered statistically significant.
Desorption kinetics
Oil extraction was conducted using the above-mentioned method using optimum soil-to-oil ratio and extraction temperature, and the desorption kinetics were studied by sampling at day 1, 2, 3, 4 and 5.
The empirical first-order mass transfer model proposed by Woolgar et al. (1999), was used to fit the desorption data according to
where C0 is the PAH concentration in the oil phase at time t (mg/L), k is the lumped mass transfer coefficient (day−1), Ce is the equilibrium oil phase PAH concentration (mg/L), and t is the contact time with oil (days).
Results and discussion
Optimisation of oil extraction parameters
Response surfaces were used to identify optimal conditions for the three independent variables using the PAH concentration as the dependent variable. Response variables were fitted with quadratic models (Text S3). Contour and three-dimensional plots for the factorial design show that PAH extraction efficiencies are highly dependent on the temperature, volume of oil, shaking period, and the matrix (Fig. S1-S4). The F-tests conducted for analysis of variance indicate the models are significant (P < 0.05) and adequately predict the extraction efficiencies.
Phenanthrene extraction from sediment was only slightly influenced by variation of extraction conditions, with the model predicting a maximum of 85% recovered using 20 °C, 5 mL of oil and a five day shaking period (Fig. S1). On the other hand, oil extraction of phenanthrene from soil was significantly (P < 0.05) influenced by temperature and shaking period. Temperatures ranging from 15 to 25 °C showed over 80% extraction of phenanthrene under different shaking periods. Nevertheless, at temperatures of 16–22 °C, longer extraction time is favoured, whereas shorter shaking period is preferred at temperatures over 22 °C, which may be due to a higher rate of phenanthrene diffusivity resulting from lower viscosity at increased temperatures (Ye et al. 2015). Volume changes had no effect on phenanthrene recovery, regardless of extraction temperature. A pairwise comparison of shaking period and oil volume (Fig. S1b) demonstrates above 95% phenanthrene recovery, which suggests phenanthrene has not saturated within the selected volume range and has no solubility issues. Accordingly, 20 °C, 6 mL of oil and seven day shaking period are the best conditions (Table S3) for phenanthrene extraction from soil.
Pyrene extraction from soil was less impacted by the variations in temperature with solvent volume than sediment. However, when temperature is compared with shaking period, maximum recoveries are found at low temperature and short shaking period in both matrices. A three day shaking period at 15 °C in soil and a five day shaking period at 20 °C in sediment provided optimal extraction (Fig. S2). All pyrene extraction efficiencies were over 80% within the tested temperature range in both soil and sediment. Extraction efficiencies decreased with increasing volume and shaking period in both matrices. Further, the use of five and three day shaking period provided optimal extraction conditions (Table S3) for pyrene in sediment and soil, respectively, when using 5 mL oil. According to the results, extraction of pyrene was more efficient from soil than sediment.
Chrysene extraction from sediment was more impacted by the interaction of the oil volume with temperature and shaking period than for soil (Fig. S3). Better recoveries of chrysene in sediment were observed when using a larger oil volume. These observations can be correlated with the relatively higher octanol partition coefficient of chrysene (Tillner et al. 2013), favouring higher volumes of hydrophobic solvent when it is bound to sediment containing less organic matter. Moreover, sediment organic matter has been reported to be less polar than that in soil (Hiller et al. 2008), and can be less soluble in oil compared to soil organic matter, limiting the release of PAHs to the oil phase. A lower temperature (15 °C) is favoured with shaking periods of three days for soil and five days for sediment.
Benzo[a]pyrene showed the highest extraction efficiency at 20 °C in both matrices, when the volume of oil is 5 mL, and the shaking period is five days (Fig. S4). In contrast, 15 °C is the most favourable temperature when using a 6–7 day shaking period, indicating that the extraction time can be reduced by increasing the temperature. Accordingly, desorption of benzo[a]pyrene suggests being governed by equilibrium conditions or mass transfer limitations, which could be altered by varying temperature, rather than by varying the solvent volume.
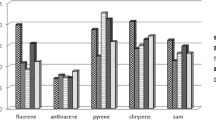
Results showed (Fig. 2) recoveries were higher in soil than in sediment, which may be due to the soil containing twelve times more organic carbon than the sediment. The sediment used in this study also contained approximately 30% more clay than the soil, which may bind with organic matter and restrict its access to oil, limiting the extraction of more recalcitrant PAHs.
Although this is the first study that uses a chemometric approach to optimize an oil extraction conditions for PAHs from soil/sediment, other studies have investigated the effect of extraction temperature, time and oil-to-soil ratio (Gong et al. 2005a; Lau et al. 2014; Pannu et al. 2004). Lau et al. (2012) studied the extraction of phenanthrene and fluoranthene from sand using soybean oil and palm kernel oil at 30 °C and 70 °C, using a 1:1 soil-to-oil ratio for 24 h. However, the study did not consider temperatures less than 30 °C, shorter extraction periods, nor the oil volume. PAH extraction efficiencies were less than 75%, even at 70 °C for either oil. Likewise, extraction efficiencies of ten PAHs ranged from 38 to 86% at 20–60 °C when extracting with peanut oil (Pannu et al. 2004). Poor recoveries from soil may be impacted by soil moisture, as recoveries have been reported to decrease by more than a third when soil is moist (Gong et al. 2005b), and may be a result of the lower porosity in moist soil compared to dried soil.
The extraction parameters chosen for a multi-analyte mix are usually compromised to provide adequate recovery of all analytes, and generally not optimal for a given PAH. This can be exacerbated by differences in the complexity of sample matrices, such as the difference between soil and sediment. The optimized conditions in the current study for the four PAHs in two different matrices suggest that the four PAHs behaved differently during oil extractions. Mechanisms such as intra-organic matter diffusion and intra-particulate diffusion may retard the desorption of PAHs sequestered in soils and sediments (Northcott et al. 2000). Plant oils may accelerate the rate of desorption of PAHs by altering such mechanisms, whereas the rate of acceleration might differ depending on the organic matter and clay composition in the matrix and the particle size. Hence, to analyse PAHs for the purpose of quantification, it is vital to have optimum extraction conditions for a few individual PAHs, which can represent the whole spectrum of PAHs or at least for few groups of PAHs categorized based on the number of rings.
Verification of the optimized method
Optimal extraction conditions were used to verify the adequacy of the model equations (Table S3). The results for phenanthrene, pyrene, and chrysene in sediment, and phenanthrene, pyrene and benzo[a]pyrene in soil matched with the predicted results, which indicated the polynomial models gave good correlations among independent variables and responses. Nonetheless, for benzo[a]pyrene in sediment and chrysene in soil, there is a significant reduction (P < 0.05) in the predicted value compared to the experimental value. However, the closeness of experimental and predicted values (< 20%) suggests the robustness of this method. Similar extraction efficiencies (80–100%) were obtained even when the extractions were performed under optimized conditions that were common for all the four PAHs in sediment (temperature: 20 °C, volume:5 mL, shaking period:5 days). Likewise, all PAHs in soil demonstrated 88–100% extraction efficiencies under the common extraction conditions.
Validation of the oil extraction method
The linearity, limits of detection, limits of quantification and recovery efficiencies were used to validate the proposed method under the optimized oil extraction conditions. Limits of detection for phenanthrene, pyrene, chrysene and benzo[a]pyrene were 16, 31, 63 and 63 µg/kg, respectively, for sediment, while the limits of detection in soil were 16 µg/kg for phenanthrene, pyrene and benzo[a]pyrene and 31 µg/kg for chrysene. The limits of quantification were 63 µg/kg for phenanthrene and pyrene and 125 µg/kg for chrysene and benzo[a]pyrene in sediment, and 63 µg/kg for phenanthrene, pyrene and benzo[a]pyrene and 125 µg/kg for chrysene in soil. Calibration plots (0.5, 1, 2, 5, 10 mg/kg) based on analyte peak areas were linear, with R2 coefficients of at least 0.999 for all analytes in soil and sediment. All four PAHs demonstrated high recovery efficiencies (Table S4) within the 0.5–10 mg/kg concentration range, with the exception of chrysene in sediment, which was significantly low (P < 0.05) for 10 mg/kg. This reduced extraction efficiency may be due to its hydrophobic nature, which may resist desorption into the oil phase due to strong interactions with the sediment organic matter, as previously reported (Carmichael et al. 1997). Likewise, a reduction in the extraction efficiencies of anthracene from 92 to 15% when the concentration is increased from 100 to 1000 mg/kg has been reported (Pannu et al. 2004). Saturation of PAHs in the oil phase at high concentrations may also result in reduced recoveries at higher concentrations.
The proposed oil extraction method allows direct analysis of PAHs via GC–MS without additional extraction or purification steps, resulting in 77–94% recoveries in soil and sediment, even at very low PAH concentrations (0.5 mg/kg). However, the increased speed and simplicity of this approach results in poorer limit of detection and limit of quantification values than for traditional solvent extraction because it does not allow for pre-concentration of samples. None of the previously discussed studies reported the detection and quantification limits for the oil assisted extraction methods. These studies tended to focus on extremely contaminated soils (~ 15–500 mg/kg), such as manufactured gas plant sites and e-waste disposal sites, and did not validate their methods against low contamination site (soils and sediments) at PAH concentrations as low as 0.5 mg/kg. Thus, the proposed oil extraction method can be considered an efficient and safe approach for extracting PAHs from different soil and sediment having different organic matter contents.
Desorption kinetic modelling
Mathematical fitting of desorption kinetic curves (Fig. 3) showed the correlation coefficient (r2) of 0.94–1 for all PAHs in soil and sediment, under the assumption of negligible PAH biodegradation during the experiment. These results indicate that the desorption of PAHs into the oil phase follows first order kinetics and are consistent with results from previous research conducted by Lau et al. (2012) and Gong et al. (2005a). Accordingly, diffusion of PAHs from low contaminated soil/sediment occurs from the surface of the soil/sediment particles, dissolving directly into the oil phase, i.e. showing monophasic behaviour.
Desorption kinetics of phenanthrene (a, b), pyrene (c, d), chrysene (e, f) and benzo[a]pyrene (g, h) in sediment and soil. C0 indicates the polycyclic aromatic hydrocarbon (PAH) concentration in the oil phase at different times. Desorption of four PAHs to oil phase throughout five days follows first order kinetics and steady state equilibrium can be achieved in two to three day shaking periods for all PAHs. Equilibrium oil phase PAH concentration in organic carbon rich soil was high relative to low organic carbon contained sediment suggesting the applicability of the proposed method to extract PAHs in different matrices
A comparison of rate constants (Table S5) suggests the existence of a higher mass transfer driving force for the most hydrophobic benzo[a]pyrene (k = 1.8 day−1) in sediment, with the opposite occurring for the more hydrophilic pyrene (k = 1.05 day−1). As the Ce values for the PAHs (Table S5) show little variation, the lower values of the rate constants for the more hydrophilic, low molecular weight-PAHs are unlikely be due to differences in solubility. Indeed, based on log Kow values of 6.13, 5.86, 5.00, and 4.52 for benzo[a]pyrene, chrysene, pyrene and phenanthrene respectively (Penezić et al. 2014) and the log Kow value for eucalyptol (2.74 see above), the low molecular weight-PAHs would expected to be somewhat more soluble in eucalyptus oil than the high molecular weight-PAHs. Hence, the differences in rate constants may be due to different affinity for sediment among the PAHs. Nevertheless, a higher rate of desorption for high molecular weight-PAHs is considered advantageous since most of the conventional extraction techniques often face difficulties in extracting high molecular weight-PAHs.
There was little variation in the mass transfer rate constants in soil, with the exception of pyrene, which had a k value of 2.02 day−1, twice as high as the other PAHs (Table S5, Fig. 3). Apart from pyrene, the rate constants in soil were less than those in sediment, and this slow diffusivity may be explained by the interactions of PAHs with organic matter in the soil, which comprised a higher percentage of organic carbon relative to sediment.
No analogous studies comparing PAH extraction rates between various matrices have been reported in the literature. On the other hand, Lau et al. (2012) have investigated palm kernel oil and soybean oil as potential alternative solvents and reported higher mass transfer rate constants for the extraction of phenanthrene and fluoranthene from sand. Their rate constants are significantly higher than reported here. This could be due to the temperature, 30 °C, compared with 20 °C used in this study. Also, sand is composed of very low levels of organic matter (0.19%) and organic carbon (0.03%), which may make release of PAHs to the solvent more facile. Other factors affecting the release of PAHs from soil and sediment may include particle size, pore size, and the presence of soot or charcoal, which can adsorb PAHs due to their high surface area. It has been reported (Karapanagioti et al. 2004) that the presence of even a small amount of high surface area carbonaceous material could contribute to strong sorption of high molecular weight PAHs. Results from this study suggest that the rate of PAH extraction to oil phase is matrix dependent and, as the kinetics of one matrix are unable to explain those of another, optimum PAH extraction times need to be established for each matrix to achieve maximum efficiency.
Conclusion
The proposed oil extraction method is capable of efficiently extracting PAHs from soil and sediment with varied levels of contamination. This approach allows direct analysis of the extract without the need for hazardous industrial organic solvents and expensive extraction equipment. However, it is vital to provide the optimal conditions for efficient extraction of PAHs as oil extraction is influenced by the extraction temperature, time, and the solvent volume. Further, application of the proposed method to Environmental Protection Agency priority PAH mix is recommended. The evidence from this study suggests that the rate of mass transfer of PAHs into the eucalyptus oil is governed by the hydrophobicity of PAHs and the nature of matrix. Hence, further research should focus on the effect of the nature of the matrix on the oil extraction of PAHs. Additionally, application of the eucalyptus oil extraction method for naturally contaminated and aged soils/sediments is vital to understand the efficacy of the method.
Availability of data and material
The datasets used and/or analysed during the current study are available from the authors on reasonable request.
Code availability
Not applicable.
References
Abbas I, Badran G, Verdin A, Ledoux F, Roumié M, Courcot D, Garçon G (2018) Polycyclic aromatic hydrocarbon derivatives in airborne particulate matter: sources, analysis and toxicity. Environ Chem Lett 16:439–475. https://doi.org/10.1007/s10311-017-0697-0
Abdel-Shafy HI, Mansour MSM (2016) A review on polycyclic aromatic hydrocarbons: source, environmental impact, effect on human health and remediation. Egypt J Pet 25:107–123. https://doi.org/10.1016/j.ejpe.2015.03.011
Carmichael LM, Christman RF, Pfaender FK (1997) Desorption and mineralization kinetics of phenanthrene and chrysene in contaminated soils. Environ Sci Technol 31:126–132. https://doi.org/10.1021/es9602105
Devan PK, Mahalakshmi NV (2009) A study of the performance, emission and combustion characteristics of a compression ignition engine using methyl ester of paradise oil–eucalyptus oil blends. Appl Energy 86:675–680. https://doi.org/10.1016/j.apenergy.2008.07.008
Gong Z, Alef K, Wilke BM, Li P (2005a) Dissolution and removal of PAHs from a contaminated soil using sunflower oil. Chemosphere 58:291–298. https://doi.org/10.1016/j.chemosphere.2004.07.035
Gong Z, Wilke BM, Alef K, Li P (2005b) Influence of soil moisture on sunflower oil extraction of polycyclic aromatic hydrocarbons from a manufactured gas plant soil. Sci Total Environ 343:51–59. https://doi.org/10.1016/j.scitotenv.2004.10.010
Gong Z, Wilke BM, Alef K, Li P, Zhou Q (2006) Removal of polycyclic aromatic hydrocarbons from manufactured gas plant-contaminated soils using sunflower oil: laboratory column experiments. Chemosphere 62:780–787. https://doi.org/10.1016/j.chemosphere.2005.04.078
Guo L, Lee HK (2013) Microwave assisted extraction combined with solvent bar microextraction for one-step solvent-minimized extraction, cleanup and preconcentration of polycyclic aromatic hydrocarbons in soil samples. J Chromatogr A 1286:9–15. https://doi.org/10.1016/j.chroma.2013.02.067
Henner P, Schwartz C, Lichtfouse E (1997) Pipette Pasteur extraction : a fast, convenient, exhaustive and environmentally friendly method for the extraction of solid samples. Analusis 25:M51–M52
Hiller E, Jurkovic L, Bartal M (2008) Effect of temperature on the distribution of polycyclic aromatic hydrocarbons in soil and sediment. Soil and Water Res 3:231–240. https://doi.org/10.17221/28/2008-SWR
Kariyawasam T, Doran GS, Howitt JA, Prenzler PD (2022) Polycyclic aromatic hydrocarbon contamination in soils and sediments: Sustainable approaches for extraction and remediation. Chemosphere 291:132981. https://doi.org/10.1016/j.chemosphere.2021.132981
Lau E, Gan S, Ng HK (2012) Extraction of phenanthrene and fluoranthene from contaminated sand using palm kernel and soybean oils. J Environ Manage 107:124–130. https://doi.org/10.1016/j.jenvman.2012.04.029
Lau E, Gan S, Ng HK, Poh PE (2014) Extraction agents for the removal of polycyclic aromatic hydrocarbons (PAHs) from soil in soil washing technologies. Environ Pollut 184:640–649. https://doi.org/10.1016/j.envpol.2013.09.010
Northcott GL, Jones KC (2000) Experimental approaches and analytical techniques for determining organic compound bound residues in soil and sediment. Environ Pollut 108:19–43. https://doi.org/10.1016/S0269-7491(99)00199-2
Pannu JK, Singh A, Ward OP (2004) Vegetable oil as a contaminated soil remediation amendment: application of peanut oil for extraction of polycyclic aromatic hydrocarbons from soil. Process Biochem 39:1211–1216. https://doi.org/10.1016/S0032-9592(03)00254-1
Parales RE, Bruce NC, Schmid A, Wackett LP (2002) Biodegradation, biotransformation, and biocatalysis (b3). Appl Environ Microbiol 68:4699–4709. https://doi.org/10.1128/AEM.68.10.4699-4709.2002
Penezić A, Gašparović B, Stipaničev D, Nelson A (2014) In-situ electrochemical method for detecting freely dissolved polycyclic aromatic hydrocarbons in water. Environ Chem 11:173–180
PubChem (2022a) Compound Summary for CID 1410, Toluene. Retrieved from https://pubchem.ncbi.nim.nih.gov/compound/Toluene
PubChem (2022b) Compound Summary for CID 2758, Eucalyptol. Retrieved from https://pubchem.ncbi.nlm.nih.gov/compound/Eucalyptol
PubChem (2022c) Compound Summary for CID 8058, Hexane. Retrieved from https://pubchem.ncbi.nlm.nih.gov/compound/Hexane
Saldana MDA, Nagpal V, Guigard SE (2005) Remediation of contaminated soils using supercritical fluid extraction: a review (1994–2004). Environ Technol 26:1013–1032. https://doi.org/10.1080/09593332608618490
Small BEJ (1981) The Australian eucalyptus oil industry—an overview. Aust for 44:170–177. https://doi.org/10.1080/00049158.1981.10674310
Tillner J, Hollard C, Bach C, Rosin C, Munoz J-F, Dauchy X (2013) Simultaneous determination of polycyclic aromatic hydrocarbons and their chlorination by-products in drinking water and the coatings of water pipes by automated solid-phase microextraction followed by gas chromatography–mass spectrometry. J Chromatogr A 1315:36–46. https://doi.org/10.1016/j.chroma.2013.09.047
Wawra A, Friesl-Hanl W, Puschenreiter M, Soja G, Reichenauer T, Roithner C, Watzinger A (2018) Degradation of polycyclic aromatic hydrocarbons in a mixed contaminated soil supported by phytostabilisation, organic and inorganic soil additives. Sci Total Environ 628–629:1287–1295. https://doi.org/10.1016/j.scitotenv.2018.02.156
Wild SR, Jones KC (1995) Polynuclear aromatic hydrocarbons in the United Kingdom environment: a preliminary source inventory and budget. Environ Pollut 88:91–108. https://doi.org/10.1016/0269-7491(95)91052-M
Woolgar PJ, Jones KC (1999) Studies on the dissolution of polycyclic aromatic hydrocarbons from contaminated materials using a novel dialysis tubing experimental method. Environ Sci Technol 33:2118–2126. https://doi.org/10.1021/es980638z
Yap CL, Gan S, Ng HK (2010) Application of vegetable oils in the treatment of polycyclic aromatic hydrocarbons-contaminated soils. J Hazard Mater 177:28–41. https://doi.org/10.1016/j.jhazmat.2009.11.078
Ye M, Sun M, Wan J, Fang G, Li H, Hu F, Jiang X, Orori Kengara F (2015) Evaluation of enhanced soil washing process with tea saponin in a peanut oil-water solvent system for the extraction of PBDEs/PCBs/PAHs and heavy metals from an electronic waste site followed by vetiver grass phytoremediation. J Chem Technol Biotechnol 90:2027–2035. https://doi.org/10.1002/jctb.4512
Yu W, Kuang S, Zhao L (2013) Uptake, accumulation and translocation of polycyclic aromatic hydrocarbons by winter wheat cultured on oily sludge-amended soil. Chin J Geochem 32:295–302. https://doi.org/10.1007/s11631-013-0635-1
Acknowledgements
Thiloka Kariyawasam acknowledges Charles Sturt University for the provision of Australian Government Research Training Program Scholarship (AGRTP) for her PhD study. Authors wish to acknowledge Curtis Kalua for his assistance in experimental designing. Julia Howitt, who contributed greatly for this paper unfortunately passed away before this paper was submitted. She will be deeply missed by her loved ones.
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions. This was supported by Australian Government Research Training Program Scholarship (AGRTP).
Author information
Authors and Affiliations
Contributions
Conceptualization and methodology was done by TK, GSD, PDP and JAH. Investigation and the first draft of the manuscript was written by TK. Formal analysis was performed by TK, PDP, GSD. Writing – review & editing was done by GSD and PDP. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflicts of interest.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kariyawasam, T., Prenzler, P.D., Howitt, J.A. et al. Greener extraction of polycyclic aromatic hydrocarbons from soil and sediment using eucalyptus oil. Environ Chem Lett 20, 2757–2764 (2022). https://doi.org/10.1007/s10311-022-01467-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10311-022-01467-0