Abstract
The mainstay of status epilepticus (SE) treatment is pharmacotherapy with anti-seizure medications (ASM). In refractory status epilepticus (RSE), when additional ASM are not effective, high-dose suppressive therapy with either benzodiazepines, thiopental, phenobarbitone, or propofol is used to suppress clinical and EEG seizure activity. However, in selected eligible cases of RSE or in super-refractory cases, epilepsy surgery may be the treatment of choice to terminate SE. Here, we review epilepsy surgery including deep brain stimulation (DBS) for treatment for RSE with emphasis on special aspects of presurgical evaluation, patient selection, and outcome. We focus on surgical treatment options for patients in the acute phase of RSE, who have received high-dose suppressive therapy prior to surgery in the majority of the cases.
Zusammenfassung
Die Hauptstütze der Behandlung des Status epilepticus (SE) ist die Pharmakotherapie mit anfallssupprimierenden Medikamenten (ASM). Bei refraktärem Status epilepticus (RSE) wird zusätzlich eine Therapie mit Benzodiazepinen, Thiopental, Phenobarbital oder Propofol zur Unterdrückung der klinischen und Elektroenzephalographie(EEG)-Anfallsaktivität eingesetzt. In ausgewählten Fällen eines RSE kann jedoch die Epilepsiechirurgie die Behandlung der Wahl sein, um den SE zu beenden. Im Folgenden wird die Epilepsiechirurgie inklusiver tiefer Hirnstimulation zur Behandlung des RSE vorgestellt, wobei der Schwerpunkt auf speziellen Aspekten der präoperativen Untersuchung, der Patientenauswahl und den Ergebnissen der wenigen Studien zum Thema liegt. Im Fokus des Artikels stehen Patienten in der akuten Phase eines therapierefraktären SE, die vor einer chirurgischen Intervention bereits eine hochdosierte anfallssupprimierende Therapie erhalten haben.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Status epilepticus (SE) is one of the most common reasons for pediatric emergency presentation and intensive care unit (ICU) admissions [1, 2]. Status epilepticus is defined by the International League Against Epilepsy (ILAE) as a convulsive or focal seizure with impaired consciousness lasting 5 or 10 min, respectively, indicating the time that emergency treatment of SE should be started [3]. Refractory status epilepticus (RSE) is present when the patient fails to respond adequately to first- and second-line antiepileptic drugs [4]. The evolution from RSE to super-refractory SE (SRSE) is defined as a duration of > 24 h after administration of anesthesia or reoccurrence after anesthesia withdrawal [5].
The mainstay of therapy in order to terminate SE is the introduction of anti-seizure medication (ASM). In RSE, high-dosage suppressive therapy with benzodiazepines, phenobarbitone, propofol, and/or thiopental is administered [6]. Morbidity and mortality strongly correlate with the etiology of SE [7]. Complications related to seizure activity itself comprise apnea, aspiration, and development of hippocampal sclerosis. Furthermore, the use of cumulative high-dosage ASM and especially high-dose suppressive therapy (HDST) prompts the need for mechanical ventilation and catecholamines, thereby increasing the risk for further complications, such as sepsis, pneumonia, and cardiovascular depression [8]. Thus, the termination of the RSE is of utmost importance. This may be achieved by elimination or treatment of the underlying cause of RSE, i.e., immunotherapy in cases of autoimmune-mediated encephalitis or antimicrobial therapy in infectious encephalitis. Accordingly, epilepsy surgery should be considered in cases of RSE caused by defined and localized structural lesions.
Epilepsy surgery aims to remove the epileptogenic zone and, at the same time, to conserve the function of the surrounding healthy brain in patients with medically refractory epilepsy [9]. In selected cases, functional impairments, such as hemianopia and hemiparesis, appear to be acceptable in order to prevent further deterioration of seizures, cognition, and behavior. Epilepsy surgery is considered in 10–20% of cases of pediatric epilepsy. However, epilepsy surgery in the context of pediatric SE is rare. In two institutional series of 750 and 391 operated pediatric patients with epilepsy, only 15 and 10 patients (2.0 and 2.6%, respectively) underwent epilepsy surgery to treat RSE [10, 11]. By contrast, a recent nationwide report in Germany reported not a single surgical case in a total cohort of 483 RSE patients [12].
Clinicians seem to be reluctant to consider epilepsy surgery in RSE for several reasons: Firstly, there are limited data on the indication and outcome of surgery in RSE, and therefore only little evidence to guide this decision; secondly, the main precondition for surgery is a clearly defined underlying structural lesion and this may not be evident in some patients; finally, epilepsy surgery typically requires a detailed presurgical evaluation but the ability to perform these diagnostic procedures is often restricted in patients with RSE within an ICU setting.
Presurgical evaluation
Several presurgical diagnostic procedures help to identify both the epileptogenic zone (EZ) and eloquent brain regions. These procedures encompass among others continuous surface EEG-video monitoring (EVM), high-resolution magnetic resonance imaging (MRI), nuclear imaging such as single-photon emission computed tomography (SPECT) and positron emission tomography (PET), neuropsychological investigations, functional MRI (fMRI), diffusion tensor imaging, invasive recordings, and cortical stimulation. Not every patient undergoes all of the aforementioned investigations but especially in cases without clearly identifiable MRI lesions, the outcome of epilepsy surgery depends on the concordance of presurgical diagnostic procedures [13].
High-resolution MRI and EEG video monitoring
In the context of epilepsy surgery for RSE, the applicability of these presurgical diagnostic procedures is limited. High-resolution MRI and detection of the EEG seizure onset with concomitant seizure semiology by long-term EVM should be considered as the minimum prerequisite. Nevertheless, EEG recordings are more prone to artifacts in the ICU setting compared to recordings on EVM units. In addition, continuous EVM with a full set of electrodes is more difficult to achieve, as the ICU staff is usually not trained on maintaining high-quality continuous EEG recording. This has prompted questioning of the usefulness of EEG montages with a reduced number of electrodes in the ICU for seizure detection. Recently, the effect of a reduced number of EEG electrodes (Fp2, C4, T8, O2, FP1, C3, T7, O1, and CZ) on the number of identified seizure patterns was compared with continuous EEG standard montages [14]. In this study, the reduction in EEG electrodes decreased the sensitivity in the detection of seizure patterns from 0.76 to 0.65. The specificity remained unchanged and correct lateralization was not significantly lower in the group with reduced electrodes. Although these new applications might be useful in judging seizure frequency in the general ICU setting, standard 10–20 or if possible adapted 10–10 montages are still required in the context of presurgical work-up.
Nuclear imaging
Nuclear imaging also faces limitations when considered for cases of RSE in the ICU setting. First, the clinical condition of the patient might not allow transport within the hospital for obtaining appropriate images. Furthermore, ongoing seizure activity and propagation in SE might lead to false localization. The exact timing of the tracer injection and image acquisition is challenging in the ICU setting and in SPECT applications. Metabolic changes due to ongoing seizure activity and continuous parenteral nutrition containing glucose might interfere with FDG-PET imaging. In summary, the use of nuclear imaging methods for localizing the EZ is more challenging in the ICU setting compared to specialized EVM units. Despite these limitations, nuclear imaging has been successfully used in the presurgical evaluation of pediatric RSE cases and was especially helpful in cases with discrepancies between MRI and EEG findings [10].
Neuropsychological evaluation
A neuropsychological evaluation cannot be performed in obtunded patients with RSE. As RSE often occurs in patients with pre-existing epilepsy, a neuropsychological evaluation may have been performed prior to SE occurrence and can provide some information for further surgical strategies.
Invasive EEG recordings with intracranial electrodes are not typically applied as presurgical work-up in pediatric RSE patients [10, 11, 15, 16] but intraoperative electrocorticography (ECoG) might contribute toward further identifying the EZ in selected cases [17].
Patient selection and outcome
Data on epilepsy surgery in children originate mostly from small case series of up to 15 patients and reports of single cases. Patient selection in most case series required the diagnosis of medically refractory epilepsy and RSE not responding to HDST encompassing treatment with phenobarbitone, propofol, thiopental, and/or midazolam [6].
A comparative study of patients who underwent epilepsy surgery with and without RSE revealed that the six patients with RSE had an earlier seizure onset (mean of 1.7 years vs. 2.4 years in the non-RSE group) and underwent hemispherotomy more often than the non-RSE group (83% vs. 56%, respectively; [16]). All patients had identifiable structural MRI lesions (cortical dysplasia, Rasmussen encephalitis, and perinatal stroke). In all patients, RSE ceased after surgery and 75% of the patients were seizure free at the 2‑year follow-up.
In another survey of ten pediatric patients, RSE was terminated in all patients by surgery [15]. All of the patients required ICU care and nine needed mechanical ventilation prior to surgery. Eight of the ten patients had congruent EEG and MRI findings. The majority of patients (60%) underwent hemispherotomy. Rates of seizure freedom were reported in 70% of the case (the observation period ranged from 4 months to 6.5 years). The two patients with non-congruent EEG and MRI findings did not become seizure-free. Two out of the three patients who did not become seizure free significantly improved in their epilepsy compared with the preoperative state. There was significant presurgical morbidity, which was interpreted mainly as a result of HDST (blood pressure instability, requirement of inotropic support, pneumonia, urinary tract infection, line infection, Clostridium difficile colitis, fever of unknown origin, neutropenia, paralytic ileus, pneumothorax, and lower-extremity deep venous thrombosis).
A study of 15 patients undergoing epilepsy surgery due to RSE comprised a more heterogeneous selection of cases as almost one third of the patients did not have a clear focal structural pathology in brain imaging [10]. Consequently, congruence of EEG and MRI was poor in the majority of these patients. In all cases, RSE was ended by surgery (one patient needed re-surgery) and seizure freedom at follow-up (at least 14 months) was seen in seven patients (47%). Two out of the six patients harboring non-localizing EEG abnormalities became seizure free. Overall, 13 out of 15 patients had nuclear imaging (either an ictal SPECT or FDG-PET study) and the majority of patients had localized abnormalities, which was used to guide further intraoperative ECoG and subsequent resection.
The most recent survey reported on ten patients undergoing epilepsy surgery in the presence of RSE. Similar to previous results, patients were young at the time of seizure onset (mean of 2.7 years) and underwent hemispherotomy in the majority of cases (70%). All patient had focal MRI abnormalities. Ictal EEG and MRI concordance was 90% for correct lateralization. Seizure outcome was categorized as Engel Class Ia (completely seizure free since surgery) in 90% of the cases and also included the one patient with diffuse ictal EEG findings.
Further case reports documented noteworthy single-case experiences in special clinical constellations and included resective surgery in n-methyl aspartate receptor (NMDAR) encephalitis and a mitochondrial disorder, or other therapeutical surgical approaches such as deep brain stimulation (DBS) or corpus callosotomy (CC).
Two pediatric patients aged 9 and 5 years with febrile infection-related epilepsy syndrome (FIRES) and RSE requiring HDST underwent centromedian thalamic nuclei DBS and anakinra treatment and showed remarkably different outcomes. Clinically meaningful seizure reduction prompting ICU discharge and good long-term outcome (attending regular school besides some seizures) was reported for the 9‑year-old patient, whereas no relevant clinical improvement was observed in the other patient [18].
Another patient (9 years old) with FIRES and RSE who underwent centromedian thalamic nuclei DBS (without anakinra treatment) demonstrated considerable improvement in baseline mental status 30 days after DBS insertion and ICU discharge, although seizure freedom was not achieved [17].
Resective surgery was performed on a 7-year-old patient with refractory partial SE secondary to NMDAR antibody encephalitis [19]. Left occipital resection was performed after 3 months of HDST treatment on the basis of localized EEG seizure activity, ictal hyperperfusion (SPECT) and cortical hypometabolism (FDG-PET) in the left posterior region. Intraoperative ECoG also revealed left occipital ictal activity. Status epilepticus stopped immediately after surgery (left occipital lobectomy) and the patient was discharged from the ICU 12 days later. At follow-up after 2 years, homonymous hemianopia and dysphasia were present. The patient was able to eat on his own but needed supervision in almost all other activities. He was still on four ASM and suffered from two to three seizures per week.
A 4-year-old child underwent palliative hemispherotomy due to medically refractory epilepsia partialis continua (EPC) of the right hemisphere caused by a heterozygous POLG1 mutation (Alpers disease), which prompted some ethical concerns [20, 21]. She was ventilated due to involvement of the left diaphragm by the EPC. After right-sided hemispherotomy, EPC stopped and the patient was discharged from the ICU. She died 2 months later due to liver failure secondary to a respiratory infection.
A 9-year-old boy with RSE of unknown cause (no focal lesions were detected on high-resolution MRI, and both EEG and ictal SPECT were not lateralizing) requiring HDST underwent CC. The RSE stopped after CC. At 6 months after surgery, neurological function had recovered to the baseline before the RSE occurred [22]. Data on vagal nerve stimulation (VNS) in pediatric RSE are sparse. In a retrospective survey of 16 patients, the authors reported on a decreased risk of SE after VNS implantation [23]. However, the patients did not undergo implantation during the acute phase of SE after receiving HDST. Similar findings were provided by a systematic review of VNS in refractory and super-refractory status in both children and adults [24]. As the information about VNS regimes was inconsequent, the authors questioned whether reporting of the treatments covered the refractory phase only.
Illustrative case
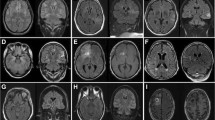
A female patient was referred for epilepsy surgery at 3.5 months of age because of medically refractory epilepsy since the first day of life due to left-sided frontotemporal cortical dysplasia (Fig. 1a–c). Long-term EEG video monitoring on the ICU showed left hemispheric seizure patterns. She received multiple trials of ASMs (valproic acid, oxcarbazepine, phenobarbitone, phenytoin, vigabatrin, levetiracetam, topiramate, lacosamide) and was placed on a ketogenic diet without significant seizure relief. Eventually, the seizures increased in number (80–160/day, Fig. 2) and evolved into RSE requiring HDST with midazolam. The need for catecholamine supply, central venous catheter, and mechanical ventilation were considered HDST-related morbidities. After left functional hemispherotomy (Fig. 3), RSE was terminated and she was finally discharged with ASM. However, 2 months after surgery, seizures reoccurred and were again refractory to ASM although there was no progression to SE. A second operation was performed 6 months after the first operation and included complete disconnection, which was not achieved during the first surgery. The patient remains seizure free since the second operation with one ASM at a follow-up period of 4 years and has shown significant neurodevelopmental gains. Surgery-related morbidity comprised right-sided spastic hemiplegia, right-sided hemianopia, and central diabetes insipidus.
A summary of red flags when considering epilepsy surgery for patients with status epilepticus is presented in the Infobox.
Infobox Summary of red flags in epilepsy surgery for patients with RSE
-
Medical refractory epilepsy
-
Need for HDST
-
Identifiable MRI lesion
-
Nuclear imaging is warranted in patients without clear structural MRI abnormalities
-
Congruence of MRI and EEG seizure activity may reflect the best outcome with respect to seizure freedom
Practical conclusion
-
Epilepsy surgery in pediatric patients with refractory status epilepticus (RSE) is rare but should be considered as an emergency treatment in eligible cases to terminate RSE, prevent complications of prolonged high-dose suppressive therapy, reduce seizure burden, and improve the neurodevelopmental prognosis.
-
In the majority of cases, RSE was terminated and seizure freedom rates of over 70% were reached in cases with clearly identifiable MRI lesions.
-
The most common surgical treatment for RSE is hemispherotomy. The outcome is favorable in patients with congruence of MRI lesions and ictal EEG activity.
-
Nuclear imaging can support localization and should be considered if MRI and EEG findings are not congruent.
-
The rarity of epilepsy surgery for RSE treatment suggests that this approach is underutilized and should be considered more frequently in eligible case.
-
Palliative attempts may also be considered but require a thorough ethical evaluation.
-
More data on deep brain stimulation are needed before recommending this method for pediatric RSE.
Abbreviations
- ASM:
-
Anti-seizure medication
- CC:
-
Corpus callosotomy
- DBS:
-
Deep brain stimulation
- ECoG:
-
Electrocorticography
- EPC:
-
Epilepsia partialis continua
- EVM:
-
Continuous surface EEG-video monitoring
- EZ:
-
Epileptogenic zone
- FIRES:
-
Febrile infection-related epilepsy syndrome
- HDST:
-
High-dose suppressive therapy
- ICU:
-
Intensive care unit
- NMDAR:
-
n-Methyl aspartate receptor
- POLG:
-
Polymerase gamma
- RSE:
-
Refractory status epilepticus
- SE:
-
Status epilepticus
- SRSE:
-
Super-refractory status epilepticus
References
Yang WC, Lin YR, Zhao LL et al (2013) Epidemiology of pediatric critically-ill patients presenting to the pediatric emergency department. Klin Padiatr 225:18–23. https://doi.org/10.1055/s-0032-1331168
Mastrangelo M, Baglioni V (2021) Management of neurological emergencies in children: an updated overview. Neuropediatrics 52:242–251. https://doi.org/10.1055/s-0041-1730936
Trinka E, Cock H, Hesdorffer D et al (2015) A definition and classification of status epilepticus—report of the ILAE task force on classification of status epilepticus. Epilepsia 56:1515–1523. https://doi.org/10.1111/epi.13121
Vasquez A, Farias-Moeller R, Tatum W (2019) Pediatric refractory and super-refractory status epilepticus. Seizure 68:62–71. https://doi.org/10.1016/j.seizure.2018.05.012
Shorvon S (2011) Super-refractory status epilepticus: an approach to therapy in this difficult clinical situation. Epilepsia 52(Suppl 8):53–56. https://doi.org/10.1111/j.1528-1167.2011.03238.x
Claassen J, Hirsch LJ, Emerson RG et al (2002) Treatment of refractory status epilepticus with pentobarbital, propofol, or midazolam: a systematic review. Epilepsia 43:146–153. https://doi.org/10.1046/j.1528-1157.2002.28501.x
Gurcharran K, Grinspan ZM (2019) The burden of pediatric status epilepticus: epidemiology, morbidity, mortality, and costs. Seizure 68:3–8. https://doi.org/10.1016/j.seizure.2018.08.021
Vasquez A, Farias-Moeller R, Sanchez-Fernandez I et al (2021) Super-refractory status epilepticus in children: a retrospective cohort study. Pediatr Crit Care Med 22:e613–e625. https://doi.org/10.1097/PCC.0000000000002786
Noachtar S, Borggraefe I (2009) Epilepsy surgery: a critical review. Epilepsy Behav 15:66–72. https://doi.org/10.1016/j.yebeh.2009.02.028
Bhatia S, Ahmad F, Miller I et al (2013) Surgical treatment of refractory status epilepticus in children. J Neurosurg Pediatr 12:360–366. https://doi.org/10.3171/2013.7.PEDS1388 (clinical article)
Jagtap SA, Kurwale N, Patil S et al (2021) Role of epilepsy surgery in refractory status epilepticus in children. Epilepsy Res 176:106744. https://doi.org/10.1016/j.eplepsyres.2021.106744
Meyer S, Langer J, Poryo M et al (2023) Epileptic status in a PEDiatric cohort (ESPED) requiring intensive care treatment: a multicenter, national, two-year prospective surveillance study. Epilepsia Open. https://doi.org/10.1002/epi4.12707
Thorsteinsdottir J, Vollmar C, Tonn JC et al (2019) Outcome after individualized stereoelectroencephalography (sEEG) implantation and navigated resection in patients with lesional and non-lesional focal epilepsy. J Neurol 266:910–920. https://doi.org/10.1007/s00415-019-09213-3
Tacke M, Janson K, Vill K et al (2022) Effects of a reduction of the number of electrodes in the EEG montage on the number of identified seizure patterns. Sci Rep 12:4621. https://doi.org/10.1038/s41598-022-08628-9
Alexopoulos A, Lachhwani DK, Gupta A et al (2005) Resective surgery to treat refractory status epilepticus in children with focal epileptogenesis. Neurology 64:567–570. https://doi.org/10.1212/01.WNL.0000150580.40019.63
Koh S, Mathern GW, Glasser G et al (2005) Status epilepticus and frequent seizures: incidence and clinical characteristics in pediatric epilepsy surgery patients. Epilepsia 46:1950–1954. https://doi.org/10.1111/j.1528-1167.2005.00340.x
Hect JL, Fernandez LD, Welch WP et al (2022) Deep brain stimulation of the centromedian thalamic nucleus for the treatment of FIRES. Epilepsia Open 7:187–193. https://doi.org/10.1002/epi4.12568
Sa M, Singh R, Pujar S et al (2019) Centromedian thalamic nuclei deep brain stimulation and anakinra treatment for FIRES—two different outcomes. Eur J Paediatr Neurol 23:749–754. https://doi.org/10.1016/j.ejpn.2019.08.001
Barros P, Brito H, Ferreira PC et al (2014) Resective surgery in the treatment of super-refractory partial status epilepticus secondary to NMDAR antibody encephalitis. Eur J Paediatr Neurol 18:449–452. https://doi.org/10.1016/j.ejpn.2014.01.013
Duchowny M (2011) Comment to the paper: palliative functional hemispherectomy for treatment of refractory status epilepticus associated with Alpers’ disease. Childs Nerv Syst 27:1327–1328. https://doi.org/10.1007/s00381-011-1501-2
Lupashko S, Malik S, Donahue D et al (2011) Palliative functional hemispherectomy for treatment of refractory status epilepticus associated with Alpers’ disease. Childs Nerv Syst 27:1321–1323. https://doi.org/10.1007/s00381-011-1495-9
Greiner HM, Tillema JM, Hallinan BE et al (2012) Corpus callosotomy for treatment of pediatric refractory status epilepticus. Seizure 21:307–309. https://doi.org/10.1016/j.seizure.2012.01.010
Gedela S, Sitwat B, Welch WP et al (2018) The effect of vagus nerve stimulator in controlling status epilepticus in children. Seizure 55:66–69. https://doi.org/10.1016/j.seizure.2018.01.010
Dibue-Adjei M, Brigo F, Yamamoto T et al (2019) Vagus nerve stimulation in refractory and super-refractory status epilepticus—a systematic review. Brain Stimul 12:1101–1110. https://doi.org/10.1016/j.brs.2019.05.011
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
I. Borggraefe, M. Tacke, M. Kunz, C. Vollmar and J. Rémi declare that they have no competing interests.
For this article no studies with human participants or animals were performed by any of the authors. All studies mentioned were in accordance with the ethical standards indicated in each case.
Additional information
Data availability statement
The data are available from the authors upon reasonable request.

Scan QR code & read article online
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Borggraefe, I., Tacke, M., Kunz, M. et al. Epilepsy surgery in pediatric refractory status epilepticus. Clin Epileptol 36, 304–309 (2023). https://doi.org/10.1007/s10309-023-00629-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10309-023-00629-6