Abstract
Seizures in neonates should prompt rapid evaluation to verify the diagnosis, determine etiology, and initiate appropriate treatment. Neonatal seizure diagnosis requires EEG confirmation and clinical observation alone is insufficient. Although most neonatal seizures are related to acute brain injury, some neonates present early-onset structural or metabolic/genetic epilepsy. Video-EEG monitoring, the gold standard for neonatal seizure detection and quantification, is resource-intensive and often unavailable, with amplitude-integrated EEG offering a reasonable alternative in guiding treatment. Whereas new-generation antiseizure medication (ASM), such as levetiracetam, appear promising, particularly in terms of tolerability, older-generation ASM, such as phenobarbital and phenytoin, are yet to be replaced. Acute treatment should aim at stopping both electroclinical and electrographic-only seizures. In neonates with acute provoked seizures, ASM should be discontinued without tapering after 72 h of seizure freedom and before hospital discharge.
Zusammenfassung
Epileptische Anfälle bei Neugeborenen sollten rasch evaluiert werden, um die Diagnose zu bestätigen, die Ursache festzustellen und eine angemessene Behandlung einzuleiten. Zur sicheren Diagnosestellung der Neugeborenenanfälle ist ein Elektroenzephalogramm (EEG) unerlässlich, da die klinische Beobachtung allein unzureichend ist. Obwohl die meisten Neugeborenenanfälle auf akute Erkrankungen des zentralen Nervensystems zurückzuführen sind, liegt bei manchen Neugeborenen eine früh beginnende strukturelle oder metabolische/genetische Epilepsie vor. Das Video-EEG-Monitoring, der Goldstandard für die Erkennung und Quantifizierung von Neugeborenenanfällen, ist ressourcenintensiv und oft nicht verfügbar, wobei das amplitudenintegrierte EEG (aEEG) eine gute Alternative in der Therapieüberwachung der Neugeborenenanfälle darstellt. Obwohl anfallsunterdrückende Medikamente der neueren Generation, wie Levetiracetam, aufgrund der besseren Verträglichkeit immer häufiger eingesetzt werden, bleiben die Medikamente der älteren Generation, wie Phenobarbital und Phenytoin, Mittel der ersten Wahl. Die Akutbehandlung sollte darauf abzielen, sowohl elektroklinische als auch rein elektrographische Anfälle anzuhalten. Bei Neugeborenen mit akut provozierten Anfällen sollte die Medikation nach 72 h Anfallsfreiheit und vor Entlassung beendet werden.
Similar content being viewed by others
Neonatal seizures are often the first and most common neurological sign in neonates. Seizures occur more frequently in the first week of life than at any other time, with a reported incidence of 1–4 in 1000 neonates, and higher rates reported in preterm neonates and in those with lower gestational age and birth weight [13]. Seizures may suggest the presence of a potentially treatable disorder and should therefore prompt a timely workup and treatment initiation [14, 18]. Neonatal seizures, notably recurrent or prolonged, can cause further brain injury, thus increasing the risk for poor neurological outcomes, including cognitive and motor impairment and post-neonatal epilepsy [14, 16]. Recent advances in diagnostics, including full EEG, amplitude-integrated EEG (aEEG), magnetic resonance imaging, metabolic and genetic testing, have drastically improved seizure detection and facilitated etiologic classification, paving the way for targeted treatment and potentially optimal outcomes. However, despite the integration of ground-breaking preclinical research and the implementation of cutting-edge technology in clinical research, treating neonatal seizures still poses a challenge for clinicians.
Etiology
Neonatal seizures are unique since most relate to acute brain injuries such as hypoxic–ischemic encephalopathy (HIE), structural brain injury—including ischemic and hemorrhagic stroke-and metabolic derangements, usually of glucose and electrolytes—and central nervous system or systemic infections. Intraventricular hemorrhage in premature neonates and HIE in term neonates are the predominant etiology of acute provoked seizures. Only ~15% of neonatal seizures are related to neonatal-onset epilepsy [14, 18] that may arise from structural brain abnormalities (structural epilepsies), such as malformations of cortical development, or genetic conditions (genetic epilepsies), such as ion channel and vitamin-dependent disorders [28]. Recurrent seizures in otherwise healthy neonates with overall negative findings may correspond to self-limited neonatal epilepsy with an excellent prognosis, whereas recurrent seizures in severely affected neonates with neurological deficit may indicate an early infantile developmental and epileptic encephalopathy with particularly poor prognosis.
Diagnostic options
Electroencephalography
Although neonatal seizures have been commonly diagnosed based on clinical semiology alone, recent studies have shown that EEG confirmation is essential to avoid misdiagnosis and guide treatment. Not all clinically suspicious events in preterm or term neonates correspond to seizures and most neonatal seizures are electrographic-only. The high rate of uncoupling, meaning that electroclinical seizures become electrographic-only following the administration of anti-seizure medication (ASM)—notably phenobarbital and phenytoin—in affected neonates, adds to this challenge [3, 20, 27].
The American Clinical Neurophysiology Society (ACNS) has defined electrographic neonatal seizures as sudden, abnormal EEG events defined by a repetitive and evolving pattern with a voltage of > 2 μV and a duration of > 10 s. “Evolving” is defined as an unequivocal evolution in frequency, voltage, morphology, or location, i.e., increasing amplitude and decreasing frequency of discharges over time [26]. When these EEG changes are related to simultaneously coupled definite clinical signs, the seizure is defined as electro-clinical; otherwise, the seizure is defined as electrographic-only. However, the definition of neonatal seizures has been reconsidered, and their duration is currently not strictly defined but has to be sufficient to (1) demonstrate an evolution in frequency and morphology of the discharges and (2) allow for the recognition of onset, evolution, and resolution of an abnormal discharge [15], with the exception of myoclonic seizures and spasms. Interestingly, EEG seizure patterns in neonates often show a focal onset and evolution, although not necessarily reflecting a focal pathology [18]. Although no consensus definition of neonatal status epilepticus is yet available [15], this may be diagnosed when the summed duration of seizures comprises ≥ 50% of an arbitrarily defined 1‑h epoch.
Continuous video EEG (v-EEG) is the gold standard for reliable neonatal seizure detection, prompt treatment initiation, and monitoring. For neonates at high risk of seizures, the ACNS recommends v‑EEG for 24 h [23]. In a prospective cohort study of neonates with clinically suspicious events or electrographic seizures who underwent v‑EEG, the median time from the beginning of the recording to electrographic seizure detection was 7 h [9]. If the EEG background activity is stable and no seizures have occurred in 24 h, the monitoring may be discontinued, except for neonates with HIE, who have a particularly high risk of seizures and thus require v‑EEG throughout cooling and rewarming. An integrative approach to neuromonitoring in high-risk neonates with v‑EEG and, ideally, a multicamera setup instead of the standard single-camera can significantly increase diagnostic accuracy. However, v‑EEG is expensive, time-consuming, and moderately invasive, while its rapid installation and real-time interpretation are prohibitive since it would require the 24/7 availability of skilled technical and medical personnel [16, 18]. To address this issue, a flexible frame of recommendations for neonatal units, encompassing different levels of complexity, local resources, and patient features, has been recently proposed [5]. Novel automated seizure detection algorithms and seizure-burden analyses developed for use in clinical settings may enhance seizure detection and provide decision support. Although no automated algorithm is reliable enough to replace experienced EEG specialists, future clinical trials will undoubtedly profit from these technological developments.
Amplitude-integrated EEG
Amplitude-integrated EEG (aEEG), with its time-compressed, one-or two-channel EEG trend presented on a semi-logarithmic scale, is increasingly applied as an adjuvant tool for continuous monitoring of affected neonates. Particularly in HIE, aEEG is an adjunctive criterion for the introduction of therapeutic hypothermia. Reducing electrode placement and interpretation time compared to conventional EEG, aEEG enables the assessment of the background activity and facilitates the earlier recognition of state changes. However, aEEG is considerably less accurate than conventional EEG for seizure detection, especially for seizures (1) arising outside the centro-temporal regions, (2) featuring a short duration, and (3) consisting of low-voltage slow discharges. However, seizures are generally more common in the central regions, and these seizure patterns can be correctly identified in 70–80% of cases since they are sampled by aEEG [24]. Although seizure detection by aEEG has been shown to miss 20% of neonatal seizures compared to full EEG, even when used by aEEG experts [25], aEEG-based seizure detection is still more reliable than clinical diagnosis alone [8]. Seizures can be detected in the aEEG as “saw-tooth-like” augmentations of the baseline amplitude but should be confirmed by examining the simultaneous EEG trace to rule out artifacts that pollute these unattended EEG recordings without simultaneous video.
Semiology
Seizure semiology can reveal specific etiologies (Table 1). Myoclonic seizures should raise suspicions of a metabolic disorder such as non-ketotic hyperglycinemia, propionic acidemia, and vitamin B6-dependent epilepsy. Focal motor clonic seizures are more frequently acute provoked, corresponding to a focal cortical lesion such as stroke, intracranial hemorrhage, and, more rarely, cortical dysplasia [19]. Motor tonic seizures often arise in genetically determined developmental epileptic encephalopathy (KCNQ2, CDKL5, STXBP1-related epilepsy, etc.) if a cortical malformation or an acute provoked etiology is unlikely based on clinical history. Sequences of a tonic followed by a myoclonic or clonic phase (sequential seizures) are a hallmark of KCNQ2-related epilepsy, the most common neonatal-onset genetic epilepsy. Epileptic spasms in neonates are rare, primarily found in metabolic disorders, such as vitamin B6-dependent epilepsy but also in cortical malformations or early-onset epileptic encephalopathy.
Classification
The Task Force on Neonatal Seizures established by the International League Against Epilepsy (ILAE) has recently introduced a diagnostic framework consisting of four domains: clinical presentation (high risk or suspicious clinical events), diagnosis (with EEG), manifestation (with or without clinical signs), and seizure types determined by the predominant clinical signs (motor: automatisms, clonic, epileptic spasms, myoclonic, sequential, and tonic; non-motor: autonomic and behavioral arrest; and unclassified) or without clinical signs [15]. In contrast to older classifications that derived from clinical semiology alone, this new classification emphasizes the critical role of EEG in the diagnosis of neonatal seizures. Paroxysmal clinical events without EEG confirmation are considered non-epileptic, and EEG-confirmed seizures are divided into electroclinical or electrographic-only seizures. Notably, neonatal seizures are generally considered focal at onset, although seizures such as spasms or myoclonic seizures may rapidly engage bilaterally distributed networks.
Differential diagnosis
The clinical differentiation of neonatal seizures from paroxysmal non-epileptic motor phenomena is particularly challenging if v‑EEG is unavailable. There is a wide range of abnormal and paroxysmal motor phenomena in neonates: tremor and jitteriness, benign neonatal sleep myoclonus, startle reflex, ocular movement disorders, paroxysmal dystonia, bilateral tonic stiffening, and hyperekplexia. Some clinical features may facilitate the differential diagnosis, including stimulus sensitivity (e.g., different stimuli, mainly auditory, for startle reflex, crying and stress for tremor and jitteriness, sudden visual stimuli or movement for paroxysmal tonic up/downward gaze), habituation (present in startle reflex but absent in hyperekplexia), and association with behavioral states (benign neonatal sleep myoclonus stops with arousal). Holding the affected limbs or repositioning the neonate can differentiate some paroxysmal non-epileptic from epileptic phenomena: Gentle restraint stops physiological tremors but does not influence epileptic events. The use of polygraphic v‑EEG is fundamental in uncovering these clinical conditions. Finally, non-epileptic paroxysmal events in the neonate are often symptomatic of an underlying pathology and should be evaluated just as systematically and thoroughly as epileptic seizures. Most abrupt changes in vital signs, such as blood pressure, heart rate, and respirations, recorded in neonates do not correspond to epileptic seizures; when these changes correspond to seizures, they are usually associated with motor phenomena or other clinical signs. In a retrospective v‑EEG study evaluating abrupt changes in vital signs, these were more likely to correspond to epileptic seizures when oxygen desaturation or apnea occurred, particularly in the presence of abnormal eye movements or abrupt tone changes [4].
Treatment
Symptomatic treatment
Starting anti-seizure medication
Particularly in HIE, both preclinical and clinical studies have provided evidence that prolonged or recurrent seizures themselves may augment injury to the developing brain beyond that of the underlying etiology [16]. For electrographic seizures, the most common seizure type in neonates, a recent study showed that those treated within 1 h of seizure onset had a significantly lower seizure burden and fewer seizures over the subsequent 24 h. This effect was absent if ASM was started > 1 h from seizure onset, suggesting that the impact of ASM on seizure burden is time-critical, with an optimal efficacy within 1 h of seizure onset. Despite this recent evidence, the optimal timepoint for treatment initiation in neonatal seizures remains ill-defined, whereas a cumulative electrographic seizure burden of > 30 s/h has been proposed as the entry criterion for randomization in therapeutic trials for neonatal seizures.
Medication selection
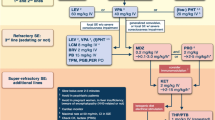
The optimal ASM management of neonatal seizures is still under debate. In a 2004 Cochrane review [2], the authors concluded that “there is little evidence from randomized controlled trials to support the use of any of the anticonvulsants currently used in the neonatal period.” Although neonatal seizure management pathways differ between institutions and settings, the most commonly used ASMs are phenobarbital (PB), phenytoin (PHT), and levetiracetam (LEV), with alternatives including lacosamide (LCM), and escalation to continuous midazolam (MDZ) infusion [11]. However, overall response rates to ASM are low, whereas the self-limited nature of acute provoked seizures and the potential neurotoxicity of ASM to the immature brain dictate caution. Standardized treatment protocols assessing ASM response in neonatal seizures may be crucial for improving outcomes and reducing adverse effects, morbidity, and mortality. Reassessing intravenous ASM efficacy 60 min from initiation has been linked to lower status epilepticus rates, lower PB concentrations, and shorter hospital stays.
The oldest and most popular first-line ASM, PB displays efficacy in only half of the cases and produces the phenomenon of electroclinical uncoupling in treated neonates. Moreover, PB has been associated with widespread neuronal apoptosis [1] and impaired synaptic maturation [7] in the animal model. It should be noted that these studies have been performed on heathy rodents and their results have neither been confirmed in humans nor has the benefit–risk ratio been evaluated in immature rodents with seizures. The main adverse effects of PB, including hypotension, respiratory suppression, and sedation, are particularly relevant for neonates with severe encephalopathy. Third-generation ASMs with a good efficacy and safety profile, particularly LEV, have emerged as novel treatment options for neonatal seizures [17]. One of the key arguments for LEV use in neonatal seizures is the more favorable pharmacokinetics, characterized by a linear clearance, few drug interactions, and a broad therapeutic index. By contrast, PB is linked to auto-inducible clearance with use and numerous drug interactions. However, PB was considerably more effective than LEV for treating neonatal seizures in a recent randomized controlled study, although higher rates of adverse effects were seen with PB [22].
Among second-line ASM, it should be noted that lidocaine reached a response rate of 68% in full-term neonates, higher than MDZ. Concerns regarding lidocaine toxicity, mainly of cardiac arrhythmias, have been eased following the introduction of new reduced-dose regimens. Nevertheless, given these potential cardiac side effects, lidocaine should not be combined with other cardiotoxic agents, e.g., phenytoin/fosphenytoin (PHT/FPHT). Little is known about the use of newer ASM, such as brivaracetam, in the neonatal period, while other, older ASMs have limited use in the acute phase because of the unavailability of an intravenous formulation. No specific ASM is indicated for preterm neonates, despite the vast differences in pharmacokinetics and brain maturation.
Finally, therapeutic hypothermia in HIE may alter the metabolism of ASM, particularly in neonates with multiorgan dysfunction. Hypothermia prolongs clearance of PHT with increased risk of bradycardia and of lidocaine with increased risk of cardiotoxicity. By contrast, the rewarming phase of hypothermia may accelerate ASM metabolism.
Etiologic treatment
The choice of treatment should be guided by the etiology of neonatal seizures since, in some cases, specific and efficacious treatment choices are available for affected neonates [14, 18]. Inborn errors of metabolism, diagnosed based on clinical presentation as well as biochemical investigations and verified by genetics, represent a significant challenge that needs to be rapidly addressed to avoid metabolic decompensation and facilitate prognostic counseling. Since early diagnosis enables precision medicine, i.e., etiologic treatment in selected metabolic disorders and specific ASM in selected neonatal-onset genetic epilepsies [12, 21], a diagnostic algorithm designed to detect these disease entities is required in all neonatal units. This algorithm should include a standardized and well-documented vitamin B6 trial to identify defects in the ALDH7A1, PNPO, PLPBP gene or cases of severe congenital hypophosphatasia [18]. Finally, some early-onset genetic epilepsies may respond to sodium channel blockers such as carbamazepine, especially loss-of-function-KCNQ2 or gain-of-function SCN2A/SCN8A. Clinical data regarding the use of quinidine in gain-of-function KCNT1 are, however, inconclusive [6].
Treatment duration
Since neonatal seizures are acute provoked in their majority, it has been suggested to wean medication to a single ASM before discharge or even withdraw ASM altogether when (1) only single or rare seizures have occurred, (2) the neonate has been seizure-free for at least 48–72 h, and (3) the risk of recurrence is not considered particularly high. Discontinuation of ASM prior to hospital discharge has not been related to a higher risk of post-neonatal epilepsy or less favorable neurodevelopmental outcomes at the 2‑year follow-up. Therefore, while the World Health Organization recommends withdrawing ASM after 72 h of seizure freedom in those with normal EEG and neurological examination [29], others suggest expanding this recommendation to neonates with abnormal EEG and neurological examination [10]. However, if seizures are uncontrolled or neonatal-onset epilepsy has been diagnosed [14], ASM should be maintained and neonates should be referred to specialized neuropediatric clinics for further management.
Practical conclusion
-
Neonatal seizures are a medical emergency and should prompt rapid evaluation to determine the etiology and introduce symptomatic and/or etiologic therapy.
-
Treatment of the underlying cause (hypoxic–ischemic encephalopathy, infection, metabolic derangements, etc.) is critical for preventing further damage to the developing brain. Although video EEG is the gold standard for neonatal seizure detection and treatment surveillance, it is resource-intensive and often unavailable, with amplitude-integrated EEG offering a reasonable alternative to guide therapeutic decisions.
-
Whereas new-generation antiseizure medications (ASMs) appear promising, particularly in terms of tolerability, these have not yet replaced older-generation ASM. Acute treatment should aim at stopping both electroclinical and electrographic-only seizures, and ASM should be given in a predefined order and in sufficient dosages to achieve plasma levels in the higher therapeutic range, provided that the ASM is well tolerated.
-
In addition to efficacy, ASM choice should be guided by the potential adverse effects of the ASM and the state of the patient since renal, cardiovascular, and hepatic dysfunction are common.
-
In neonates with acute provoked seizures, ASM should be discontinued without tapering after 72 h of seizure freedom and before hospital discharge. Neonates with neonatal-onset epilepsy should be maintained on ASM and followed up closely in specialized neuropediatric clinics.
-
While diagnosing and treating neonatal seizures remains challenging, the development of novel, disease-modifying, or anti-epileptogenic therapies and new neuroprotective agents may ultimately improve neonatal seizure outcomes.
References
Bittigau P, Sifringer M, Genz K et al (2002) Antiepileptic drugs and apoptotic neurodegeneration in the developing brain. Proc Natl Acad Sci U S A 99:15089–15094. https://doi.org/10.1073/pnas.222550499
Booth D, Evans DJ (2004) Anticonvulsants for neonates with seizures. Cochrane Database Syst Rev. https://doi.org/10.1002/14651858.CD004218.pub2
Boylan GB, Stevenson NJ, Vanhatalo S (2013) Monitoring neonatal seizures. Semin Fetal Neonatal Med 18:202–208. https://doi.org/10.1016/j.siny.2013.04.004
Dang LT, Shellhaas RA (2016) Diagnostic yield of continuous video electroencephalography for paroxysmal vital sign changes in pediatric patients. Epilepsia 57:272–278. https://doi.org/10.1111/epi.13276
Dilena R, Raviglione F, Cantalupo G et al (2021) Consensus protocol for EEG and amplitude-integrated EEG assessment and monitoring in neonates. Clin Neurophysiol 132:886–903. https://doi.org/10.1016/j.clinph.2021.01.012
Fitzgerald MP, Fiannacca M, Smith DM et al (2019) Treatment responsiveness in KCNT1-related epilepsy. Neurother J Am Soc Exp Neurother 16:848–857. https://doi.org/10.1007/s13311-019-00739-y
Forcelli PA, Janssen MJ, Vicini S, Gale K (2012) Neonatal exposure to antiepileptic drugs disrupts striatal synaptic development. Ann Neurol 72:363–372. https://doi.org/10.1002/ana.23600
Frenkel N, Friger M, Meledin I et al (2011) Neonatal seizure recognition—comparative study of continuous-amplitude integrated EEG versus short conventional EEG recordings. Clin Neurophysiol 122:1091–1097. https://doi.org/10.1016/j.clinph.2010.09.028
Glass HC, Shellhaas RA, Wusthoff CJ et al (2016) Contemporary profile of seizures in neonates: a prospective cohort study. J Pediatr 174:98–103.e1. https://doi.org/10.1016/j.jpeds.2016.03.035
Glass HC, Soul JS, Chang T et al (2021) Safety of early discontinuation of Antiseizure medication after acute symptomatic neonatal seizures. JAMA Neurol 78:817–825. https://doi.org/10.1001/jamaneurol.2021.1437
Keene JC, Morgan LA, Abend NS et al (2021) Treatment of neonatal seizures: comparison of treatment pathways from 11 neonatal intensive care units. Pediatr Neurol. https://doi.org/10.1016/j.pediatrneurol.2021.10.004
Numis AL, Angriman M, Sullivan JE et al (2014) KCNQ2 encephalopathy: delineation of the electroclinical phenotype and treatment response. Neurology 82:368–370. https://doi.org/10.1212/WNL.0000000000000060
Pisani F, Facini C, Bianchi E et al (2018) Incidence of neonatal seizures, perinatal risk factors for epilepsy and mortality after neonatal seizures in the province of Parma, Italy. Epilepsia 59:1764–1773. https://doi.org/10.1111/epi.14537
Pisani F, Spagnoli C, Falsaperla R et al (2021) Seizures in the neonate: a review of etiologies and outcomes. Seizure 85:48–56. https://doi.org/10.1016/j.seizure.2020.12.023
Pressler RM, Cilio MR, Mizrahi EM et al (2021) The ILAE classification of seizures and the epilepsies: modification for seizures in the neonate. Position paper by the ILAE task force on neonatal seizures. Epilepsia 62:615–628. https://doi.org/10.1111/epi.16815
Ramantani G (2013) Neonatal epilepsy and underlying aetiology: to what extent do seizures and EEG abnormalities influence outcome? Epileptic Disord 15:365–375. https://doi.org/10.1684/epd.2013.0619
Ramantani G, Ikonomidou C, Walter B et al (2011) Levetiracetam: safety and efficacy in neonatal seizures. Eur J Paediatr Neurol 15:1–7. https://doi.org/10.1016/j.ejpn.2010.10.003
Ramantani G, Schmitt B, Plecko B et al (2019) Neonatal seizures-are we there yet? Neuropediatrics 50:280–293. https://doi.org/10.1055/s-0039-1693149
Santarone ME, Pietrafusa N, Fusco L (2020) Neonatal seizures: when semiology points to etiology. Seizure 80:161–165. https://doi.org/10.1016/j.seizure.2020.06.025
Scher MS, Alvin J, Gaus L et al (2003) Uncoupling of EEG-clinical neonatal seizures after antiepileptic drug use. Pediatr Neurol 28:277–280
Schubert-Bast S, Hofstetter P, Fischer D et al (2017) Sodium channel blockers in KCNQ2-encephalopathy: lacosamide as a new treatment option. Seizure 51:171–173. https://doi.org/10.1016/j.seizure.2017.08.005
Sharpe C, Reiner GE, Davis SL et al (2020) Levetiracetam versus phenobarbital for neonatal seizures: a randomized controlled trial. Pediatrics 145:e20193182. https://doi.org/10.1542/peds.2019-3182
Shellhaas RA, Chang T, Tsuchida T et al (2011) The American clinical neurophysiology society’s guideline on continuous electroencephalography monitoring in neonates. J Clin Neurophysiol 28:611–617
Shellhaas RA, Soaita AI, Clancy RR (2007) Sensitivity of amplitude-integrated electroencephalography for neonatal seizure detection. Pediatrics 120:770–777. https://doi.org/10.1542/peds.2007-0514
Toet MC, van der Meij W, de Vries LS et al (2002) Comparison between simultaneously recorded amplitude integrated electroencephalogram (cerebral function monitor) and standard electroencephalogram in neonates. Pediatrics 109:772–779
Tsuchida TN, Wusthoff CJ, Shellhaas RA et al (2013) American clinical neurophysiology society standardized EEG terminology and categorization for the description of continuous EEG monitoring in neonates: report of the American Clinical Neurophysiology Society critical care monitoring committee. J Clin Neurophysiol 30:161–173. https://doi.org/10.1097/WNP.0b013e3182872b24
Weiner SP, Painter MJ, Geva D et al (1991) Neonatal seizures: electroclinical dissociation. Pediatr Neurol 7:363–368. https://doi.org/10.1016/0887-8994(91)90067-u
Zuberi SM, Wirrell E, Yozawitz E et al (2022) ILAE classification and definition of epilepsy syndromes with onset in neonates and infants: position statement by the ILAE Task Force on Nosology and Definitions. Epilepsia 63:1349–1397. https://doi.org/10.1111/epi.17239
WHO (2011) Guidelines on Neonatal Seizures. World Health Organization, Geneva
Acknowledgements
We thank the Vontobel Foundation (to G.R.) for funding.
Funding
INFORMATION: Vontobel-Stiftung, Prof. Dr. Georgia RAMANTANI
Funding
Open access funding provided by University of Zurich
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
G. Ramantani and F. Pisani declare that they have no competing interests.
For this article no studies with human participants or animals were performed by any of the authors. All studies mentioned were in accordance with the ethical standards indicated in each case.
Additional information

Scan QR code & read article online
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ramantani, G., Pisani, F. Neonatal seizures—diagnostic options and treatment recommendations. Z. Epileptol. 35, 310–316 (2022). https://doi.org/10.1007/s10309-022-00534-4
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10309-022-00534-4