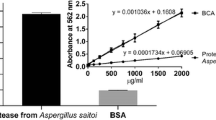
Abstract
Protease-secreting yeasts have broad biotechnological potential for application to various industrial processes, including winemaking. However, this activity is influenced by the yeast response to environmental factors such as nitrogen and protein sources, as are found in grape juice. In this study, the wine-relevant yeast Metschnikowia pulcherrima IWBT Y1123, with known protease-secreting ability, was subjected to different nitrogen-containing compounds to monitor their impact on protease secretion and activity. Protease activity increased above basal levels for haemoglobin-containing treatments, indicating an inductive influence of proteins. On the other hand, treatments containing both haemoglobin and assimilable nitrogen sources led to a delayed increase in protease activity and protein degradation, suggesting a nitrogen catabolite repression mechanism at work. Protease activity and expression were furthermore evaluated in grape juice, which revealed increased expression and activity levels over time as promising results for further investigations into the impact of this yeast on wine properties.





Similar content being viewed by others
References
Alexandre H, Heintz D, Chassagne D, Guilloux-Benatier M, Charpentier C, Feuillat M (2001) Protease A activity and nitrogen fractions released during alcoholic fermentation and autolysis in enological conditions. J Ind Microbiol Biotechnol 26:235–240
Beltran G, Novo M, Rozès N, Mas A, Guillamón JM (2004) Nitrogen catabolite repression in Saccharomyces cerevisiae during wine fermentations. FEMS Yeast Res 4:625–632
Bolumar T, Sanz Y, Aristoy MC, Toldrá F (2006) Protease (PrA and PrB) and prolyl and arginyl aminopeptidase activities from Debaryomyces hansenii as a function of growth phase and nutrient sources. Int J Food Microbiol 107:20–26
Bolumar T, Sanz Y, Aristoy MC, Toldrá F (2008) Purification and characterisation of Proteases A and D from Debaryomyces hansenii. Int J Food Microbiol 124:135–141
Boutrou R, Kerriou L, Gassi JY (2006) Contribution of Geotrichum candidum to the proteolysis of soft cheese. Int Dairy J 16:775–783
Braaksma M, Smilde AK, van der Werf MJ, Punt PJ (2009) The effect of environmental conditions on extracellular protease activity in controlled fermentations of Aspergillus niger. Microbiology 155:3430–3439
Chaud LCS, Lario LD, Bonugli-Santos RC, Sette LD, Junior AP, Felipe MGA (2016) Improvement in extracellular protease production by the marine antarctic yeast Rhodotorula mucilaginosa L7. N Biotechnol 33:807–814
Christensen T, Hynes MJ, Davis MA (1998) Role of the regulatory gene areA of Aspergillus oryzae in nitrogen metabolism. Appl Environ Microbiol 64:3232–3237
Cohen BL, Morris JE, Drucker H (1975) Regulation of two extracellular proteases of Neurospora crassa by induction and by carbon-nitrogen and sulfur-metabolite repression. Arch Biochem Biophys 169:324–330
Dabas N, Morschhäuser J (2008) A transcription factor regulatory cascade controls secreted aspartic protease expression in Candida albicans. Mol Microbiol 69:586–602
Dunkel N, Biswas K, Hiller E, Fellenberg K, Satheesh SV, Rupp S, Morschhäuser J (2014) Control of morphogenesis, protease secretion and gene expression in Candida albicans by the preferred nitrogen source ammonium. Microbiology 160:1599–1608
Escribano R, González-Arenzana L, Garijo P, Berlanas C, López-Alfaro I, López R, Gutiérrez AR, Santamaría P (2017) Screening of enzymatic activities within different enological non-Saccharomyces yeasts. J Food Sci Technol 54:1555–1564
Farley PC, Ikasari L (1992) Regulation of the secretion of Rhizopus oligosporus extracellular carboxyl proteinase. J Gen Microbiol 138:2539–2544
Geisseler D, Horwath WR (2008) Regulation of extracellular protease activity in soil in response to different sources and concentrations of nitrogen and carbon. Soil Biol Biochem 40:3040–3048
Gente S, Poussereau N, Fèvre M (1999) Isolation and expression of a nitrogen regulatory gene, nmc, of Penicillium roqueforti. FEMS Microbiol Lett 175:291–297
Godard P, Urrestarazu A, Vissers S, Kontos K, Bontempi G, van Helden J, André B (2007) Effect of 21 different nitrogen sources on global gene expression in the yeast Saccharomyces cerevisiae. Mol Cell Biol 27:3065–3086
Henschke PA, Jiranek V (1993) Yeasts—metabolism of nitrogen compounds. In: Fleet GH (ed) Wine microbiology and biotechnology. Taylor & Francis, Cornwall, pp 77–164
Huang YX, Wu ZJ, Huang BT, Luo M (2013) Pathway and mechanism of pH dependent human hemoglobin tetramer-dimer-monomer dissociations. PLoS One 8:e81708
Kalisz HM (1988) Microbial proteinases. Adv Biochem Eng 36:1–65
Katz ME, Bernardo SM, Cheetham BF (2008) The interaction of induction, repression and starvation in the regulation of extracellular proteases in Aspergillus nidulans: evidence for a role for CreA in the response to carbon starvation. Curr Genet 54:47–55
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685
Lekkas C, Hill AE, Taidi B, Hodgson J, Stewart GG (2009) The role of small wort peptides in brewing fermentations. J Inst Brew 115:134–139
Marangon M, Van Sluyter SC, Robinson EMC, Muhlack RA, Holt HE, Haynes PA, Godden PW, Smith PA, Waters EJ (2012) Degradation of white wine haze proteins by Aspergillopepsin I and II during juice flash pasteurization. Food Chem 135:1157–1165
Maturano YP, Assaf LAR, Toro ME, Nally MC, Vallejo M, de Figueroa LIC, Combina M, Vazquez F (2012) Multi-enzyme production by pure and mixed cultures of Saccharomyces and non-Saccharomyces yeasts during wine fermentation. Int J Food Microbiol 155:43–50
McRae JM, Schulkin A, Dambergs RG, Smith PA (2018) Effect of white wine composition on protein haze potential. Aust J Grape Wine Res 24:1–6
Mendes-Ferreira A, Barbosa C, Lage P, Mendes-Faia A (2011) The impact of nitrogen on yeast fermentation and wine quality. Ciência e Técnica Vitivinic 26:17–32
Ogrydziak DM, Demain AL, Tannenbaum SR (1977) Regulation of extracellular protease production in Candida lipolytica. Biochim Biophys Acta 497:525–538
Oro L, Ciani M, Comitini F (2014) Antimicrobial activity of Metschnikowia pulcherrima on wine yeasts. J Appl Microbiol 116:1209–1217
Ray MK, Devi KU, Kumar GS, Shivaji S (1992) Extracellular protease from the antarctic yeast Candida humicola. Appl Environ Microbiol 58:1918–1923
Reid VJ, Theron LW, du Toit M, Divol B (2012) Identification and partial characterization of extracellular aspartic protease genes from Metschnikowia pulcherrima IWBT Y1123 and Candida apicola IWBT Y1384. Appl Environ Microbiol 78:6838–6849
Rutledge RG (2011) A java program for LRE-based real-time qPCR that enables large-scale absolute quantification. PLoS One 6:e17636
Rutledge RG, Stewart D (2010) Assessing the performance capabilities of LRE-based assays for absolute quantitative real-time PCR. PLoS One 5:e9731
Van Sluyter SC, McRae JM, Falconer RJ, Smith PA, Bacic A, Waters EJ, Marangon M (2015) Wine protein haze: mechanisms of formation and advances in prevention. J Agric Food Chem 63:4020–4030
Van Sluyter SC, Warnock NI, Schmidt S, Anderson P, van Kan JAL, Bacic A, Waters EJ (2013) Aspartic acid protease from Botrytis cinerea removes haze-forming proteins during white winemaking. J Agric Food Chem 61:9705–9711
Snyman C, Theron L, Divol B (2019) Understanding the regulation of extracellular protease gene expression in fungi: a key step towards their biotechnological applications. Appl Microbiol Biotechnol 103:5517–5532
Sternberg MZ (1970) The separation of proteins with heteropolyacids. Biotechnol Bioeng 12:1–17
Szopinska A, Christ E, Planchon S, König H, Evers D, Renaut J (2016) Stuck at work? Quantitative proteomics of environmental wine yeast strains reveals the natural mechanism of overcoming stuck fermentation. Proteomics 16:593–608
Teste MA, Duquenne M, François JM, Parrou JL (2009) Validation of reference genes for quantitative expression analysis by real-time RT-PCR in Saccharomyces cerevisiae. BMC Mol Biol 10:99–114
Theron LW, Bely M, Divol B (2017) Characterisation of the enzymatic properties of MpAPr1, an aspartic protease secreted by the wine yeast Metschnikowia pulcherrima. J Sci Food Agric 97:3584–3593
Theron LW, Bely M, Divol B (2018) Monitoring the impact of an aspartic protease (MpAPr1) on grape proteins and wine properties. Appl Microbiol Biotechnol 102:5173–5183
Zaman S, Lippman SI, Zhao X, Broach JR (2008) How Saccharomyces responds to nutrients. Annu Rev Genet 42:27–81
Acknowledgements
This work is based on research supported in part by the National Research Foundation of South Africa (Grant number: 113303) and Winetech for their financial support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Research involving human and animal participants
This article does not involve any studies with human participants or animals performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Snyman, C., Theron, L.W. & Divol, B. The expression, secretion and activity of the aspartic protease MpAPr1 in Metschnikowia pulcherrima IWBT Y1123. J Ind Microbiol Biotechnol 46, 1733–1743 (2019). https://doi.org/10.1007/s10295-019-02227-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10295-019-02227-w