Abstract
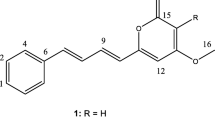
Type III polyketide synthases (PKSs) are comparatively small homodimeric enzymes affording natural products with diverse structures and functions. While type III PKS biosynthetic pathways have been studied thoroughly in plants, their counterparts from bacteria and fungi are to date scarcely characterized. This gap is exemplified by myxobacteria from which no type III PKS-derived small molecule has previously been isolated. In this study, we conducted a genomic survey of myxobacterial type III PKSs and report the identification of uncommon alkylpyrones as the products of type III PKS biosynthesis from the myxobacterial model strain Myxococcus xanthus DK1622 through a self-resistance-guided screening approach focusing on genes encoding pentapetide repeat proteins, proficient to confer resistance to topoisomerase inhibitors. Using promoter-induced gene expression in the native host as well as heterologous expression of biosynthetic type III PKS genes, sufficient amounts of material could be obtained for structural elucidation and bioactivity testing, revealing potent topoisomerase activity in vitro.







Similar content being viewed by others
References
Austin MB, Noel JP (2003) The chalcone synthase superfamily of type III polyketide synthases. Nat Prod Rep 20(1):79–110. https://doi.org/10.1039/B100917F
Austin MB, Izumikawa M, Bowman ME et al (2004) Crystal structure of a bacterial type III polyketide synthase and enzymatic control of reactive polyketide intermediates. J Biol Chem 279(43):45162–45174. https://doi.org/10.1074/jbc.M406567200
Baumann S, Herrmann J, Raju R et al (2014) Cystobactamids: myxobacterial topoisomerase inhibitors exhibiting potent antibacterial activity. Angew Chem Int Ed 53(52):14605–14609. https://doi.org/10.1002/anie.201409964
Bode HB, Ring MW, Schwär G et al (2009) Identification of additional players in the alternative biosynthesis pathway to isovaleryl-CoA in the myxobacterium Myxococcus xanthus. ChemBioChem 10(1):128–140. https://doi.org/10.1002/cbic.200800219
Chemler JA, Buchholz TJ, Geders TW et al (2012) Biochemical and structural characterization of germicidin synthase. Analysis of a type III polyketide synthase that employs acyl-ACP as a starter unit donor. J Am Chem Soc 134(17):7359–7366. https://doi.org/10.1021/ja2112228
Cociancich S, Pesic A, Petras D et al (2015) The gyrase inhibitor albicidin consists of p-aminobenzoic acids and cyanoalanine. Nat Chem Biol 11(3):195–197. https://doi.org/10.1038/nchembio.1734
Collin F, Karkare S, Maxwell A (2011) Exploiting bacterial DNA gyrase as a drug target. Current state and perspectives. Appl Microbiol Biotechnol 92(3):479–497. https://doi.org/10.1007/s00253-011-3557-z
Edayathumangalam R, Wu R, Garcia R et al (2013) Crystal structure of Bacillus subtilis GabR, an autorepressor and transcriptional activator of gabT. Proc Natl Acad Sci USA 110(44):17820–17825. https://doi.org/10.1073/pnas.1315887110
Edgar RC (2004) MUSCLE: a multiple sequence alignment method with reduced time and space complexity. BMC Bioinform 5:113. https://doi.org/10.1186/1471-2105-5-113
Edgar RC (2004) MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res 32(5):1792–1797. https://doi.org/10.1093/nar/gkh340
Finn RD, Coggill P, Eberhardt RY et al (2016) The Pfam protein families database: towards a more sustainable future. Nucleic Acids Res 44(D1):D279–285. https://doi.org/10.1093/nar/gkv1344
Funa N, Ohnishi Y, Ebizuka Y et al (2002) Properties and substrate specificity of RppA, a chalcone synthase-related polyketide synthase in Streptomyces griseus. J Biol Chem 277(7):4628–4635. https://doi.org/10.1074/jbc.M110357200
Funa N, Funabashi M, Yoshimura E et al (2005) A novel quinone-forming monooxygenase family involved in modification of aromatic polyketides. J Biol Chem 280(15):14514–14523
Funa N, Funabashi M, Ohnishi Y et al (2005) Biosynthesis of hexahydroxyperylenequinone melanin via oxidative aryl coupling by cytochrome P-450 in Streptomyces griseus. J Bacteriol 187(23):8149–8155. https://doi.org/10.1128/JB.187.23.8149-8155.2005
Funabashi M, Funa N, Horinouchi S (2008) Phenolic lipids synthesized by type III polyketide synthase confer penicillin resistance on Streptomyces griseus. J Biol Chem 283(20):13983–13991. https://doi.org/10.1074/jbc.M710461200
Gokulan K, O’Leary SE, Russell WK et al (2013) Crystal structure of Mycobacterium tuberculosis polyketide synthase 11 (PKS11) reveals intermediates in the synthesis of methyl-branched alkylpyrones. J Biol Chem 228(23):16484–16494. https://doi.org/10.1074/jbc.M113.468892
Gross F, Luniak N, Perlova O et al (2006) Bacterial type III polyketide synthases: phylogenetic analysis and potential for the production of novel secondary metabolites by heterologous expression in pseudomonads. Arch Microbiol 185(1):28–38. https://doi.org/10.1007/s00203-005-0059-3
Hashimi SM, Wall MK, Smith AB et al (2007) The phytotoxin albicidin is a novel inhibitor of DNA gyrase. Antimicrob Agents Chemother 51(1):181–187. https://doi.org/10.1128/AAC.00918-06
Hayashi T, Kitamura Y, Funa N et al (2011) Fatty acyl-AMP ligase involvement in the production of alkylresorcylic acid by a Myxococcus xanthus type III polyketide synthase. ChemBioChem 12(14):2166–2176. https://doi.org/10.1002/cbic.201100344
Hegde SS, Vetting MW, Roderick SL et al (2005) A fluoroquinolone resistance protein from Mycobacterium tuberculosis that mimics DNA. Science 308(5727):1480–1483. https://doi.org/10.1126/science.1109745
Herrmann J, Fayad AA, Müller R (2017) Natural products from myxobacteria: novel metabolites and bioactivities. Nat Prod Rep 34(2):135–160. https://doi.org/10.1039/C6NP00106H
Hoffmann T, Krug D, Hüttel S et al (2014) Improving natural products identification through targeted LC-MS/MS in an untargeted secondary metabolomics workflow. Anal Chem 86(21):10780–10788. https://doi.org/10.1021/ac502805w
Huang T, Lin S (2017) Microbial natural products. A promising source for drug discovery. Appl Microbiol Biotechnol 1(2):1–3. https://doi.org/10.21767/2576-1412.100005
Huo L, Rachid S, Stadler M et al (2012) Synthetic biotechnology to study and engineer ribosomal bottromycin biosynthesis. Chem Biol 19(10):1278–1287. https://doi.org/10.1016/j.chembiol.2012.08.013
Iniesta AA, García-Heras F, Abellón-Ruiz J et al (2012) Two systems for conditional gene expression in Myxococcus xanthus inducible by isopropyl-β-d-thiogalactopyranoside or vanillate. J Bacteriol 194(21):5875–5885. https://doi.org/10.1128/JB.01110-12
Kashefi K, Hartzell PL (1995) Genetic suppression and phenotypic masking of a Myxococcus xanthus frzF-defect. Mol Microbiol 15(3):483–494
Kelley LA, Mezulis S, Yates CM et al (2015) The Phyre2 web portal for protein modeling, prediction and analysis. Nat Protoc 10(6):845–858. https://doi.org/10.1038/nprot.2015.053
Koskiniemi H, Metsa-Ketela M, Dobritzsch D et al (2007) Crystal structures of two aromatic hydroxylases involved in the early tailoring steps of angucycline biosynthesis. J Mol Biol 372(3):633–648. https://doi.org/10.1016/j.jmb.2007.06.087
Mallika V, Sivakumar KC, Soniya EV (2011) Evolutionary implications and physicochemical analyses of selected proteins of type III polyketide synthase family. Evol Bioinform 7:41–53. https://doi.org/10.4137/EBO.S6854
Mascotti ML, Juri Ayub M, Furnham N et al (2016) Chopping and changing: the evolution of the flavin-dependent monooxygenases. J Mol Biol 428(15):3131–3146. https://doi.org/10.1016/j.jmb.2016.07.003
Minowa Y, Araki M, Kanehisa M (2007) Comprehensive analysis of distinctive polyketide and nonribosomal peptide structural motifs encoded in microbial genomes. J Mol Biol 368(5):1500–1517. https://doi.org/10.1016/j.jmb.2007.02.099
Miyanaga A, Funa N, Awakawa T et al (2008) Direct transfer of starter substrates from type I fatty acid synthase to type III polyketide synthases in phenolic lipid synthesis. Proc Natl Acad Sci USA 105(3):871–876
Morita H, Yamashita M, Shi SP et al (2011) Synthesis of unnatural alkaloid scaffolds by exploiting plant polyketide synthase. Proc Natl Acad Sci USA 108(33):13504–13509. https://doi.org/10.1073/pnas.1107782108
Ohnishi Y, Ishikawa J, Hara H et al (2008) Genome sequence of the streptomycin-producing microorganism Streptomyces griseus IFO 13350. J Bacteriol 190(11):4050–4060. https://doi.org/10.1128/JB.00204-08
Panter F, Krug D, Baumann S et al (2018) Self-resistance guided genome mining uncovers new topoisomerase inhibitors from myxobacteria. Chem Sci 9(21):4898–4908. https://doi.org/10.1039/C8SC01325J
Parvez A, Giri S, Giri GR et al (2018) Novel type III polyketide synthases biosynthesize methylated polyketides in Mycobacterium marinum. Sci Rep 8(1):6529. https://doi.org/10.1038/s41598-018-24980-1
Sambrook J, Russell DW (2001) Molecular cloning: a laboratory manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor
Sankaranarayanan R, Saxena P, Marathe UB et al (2004) A novel tunnel in mycobacterial type III polyketide synthase reveals the structural basis for generating diverse metabolites. Nat Struct Mol Biol 11(9):894–900. https://doi.org/10.1038/nsmb809
Satou R, Miyanaga A, Ozawa H et al (2013) Structural basis for cyclization specificity of two Azotobacter type III polyketide synthases. A single amino acid substitution reverses their cyclization specificity. J Biol Chem 288(47):34146–34157. https://doi.org/10.1074/jbc.m113.487272
Schuz R, Heller W, Hahlbrock K (1983) Substrate specificity of chalcone synthase from Petroselinum hortense. Formation of phloroglucinol derivatives from aliphatic substrates. J Biol Chem 258(11):6730–6734
Shah S, Heddle JG (2014) Squaring up to DNA. Pentapeptide repeat proteins and DNA mimicry. Appl Biochem Biotechnol 98(23):9545–9560. https://doi.org/10.1007/s00253-014-6151-3
Shimizu Y, Ogata H, Goto S (2017) Type III polyketide synthases. Functional classification and phylogenomics. ChemBioChem 18(1):50–65. https://doi.org/10.1002/cbic.201600522
Sone Y, Nakamura S, Sasaki M et al. (2018) Identification and characterization of bacterial enzymes catalyzing the synthesis of 1,8-dihydroxynaphthalene, a key precursor of dihydroxynaphthalene melanin, from Sorangium cellulosum. Appl Environ Microbiol. AEM-00258-18. https://doi.org/10.1128/aem.00258-18
Sucipto H, Pogorevc D, Luxenburger E et al (2017) Heterologous production of myxobacterial α-pyrone antibiotics in Myxococcus xanthus. Metab Eng 44:160–170. https://doi.org/10.1016/j.ymben.2017.10.004
Taylor JA, Burton NP, Maxwell A (2012) High-throughput microtitre plate-based assay for DNA topoisomerases. Methods Mol Biol 815:229–239. https://doi.org/10.1007/978-1-61779-424-7_17
Vetting MW, Hegde SS, Fajardo JE et al (2006) Pentapeptide repeat proteins. Biochemistry 45(1):1–10. https://doi.org/10.1021/bi052130w
Vetting MW, Hegde SS, Zhang Y et al (2011) Pentapeptide-repeat proteins that act as topoisomerase poison resistance factors have a common dimer interface. Acta Crystallogr Sect F 67(Pt 3):296–302. https://doi.org/10.1107/S1744309110053315
Wang H, Li Z, Jia R et al (2016) RecET direct cloning and Redαβ recombineering of biosynthetic gene clusters, large operons or single genes for heterologous expression. Nat Protoc 11(7):1175–1190. https://doi.org/10.1038/nprot.2016.054
Wenzel SC, Müller R (2007) Myxobacterial natural product assembly lines: fascinating examples of curious biochemistry. Nat Prod Rep 24(6):1211–1224. https://doi.org/10.1039/b706416k
Wenzel SC, Müller R (2009) The impact of genomics on the exploitation of the myxobacterial secondary metabolome. Nat Prod Rep 26(11):1385–1407. https://doi.org/10.1039/b817073h
Witte SNR, Hug JJ, Géraldy M et al (2017) Biosynthesis, and total synthesis of pyrronazol B a secondary metabolite from Nannocystis pusilla. Chem Eur J 23(63):15917–15921. https://doi.org/10.1002/chem.201703782
Yu D, Xu F, Zeng J et al (2012) Type III polyketide synthases in natural product biosynthesis. IUBMB Life 64(4):285–295. https://doi.org/10.1002/iub.1005
Zha W, Rubin-Pitel SB, Zhao H (2006) Characterization of the substrate specificity of PhlD, a type III polyketide synthase from Pseudomonas fluorescens. J Biol Chem 281(42):32036–32047. https://doi.org/10.1074/jbc.M606500200
Zhang Y, Buchholz F, Muyrers JP et al (1998) A new logic for DNA engineering using recombination in Escherichia coli. Nat Genet 20:123–128. https://doi.org/10.1038/2417
Acknowledgements
The authors thank Alexander Popoff for recording the NMR spectra of the alkylpyrones, Viktoria Schmitt and Jennifer Herrmann for performing bioactivity assays, Ronald Garcia for microbiological assistance and Nestor Zaburannyi for bioinformatic support. Joachim J. Hug was supported by a PhD fellowship of the Boehringer Ingelheim Fonds.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Dedicated to Professor Heinz G. Floss for his numerous contributions to the field of natural products.
This article is part of the Special Issue “Natural Product Discovery and Development in the Genomic Era 2019”.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Hug, J.J., Panter, F., Krug, D. et al. Genome mining reveals uncommon alkylpyrones as type III PKS products from myxobacteria. J Ind Microbiol Biotechnol 46, 319–334 (2019). https://doi.org/10.1007/s10295-018-2105-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10295-018-2105-6