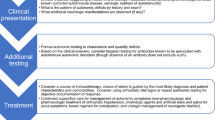
Abstract
Objectives
To review infectious diseases that may cause autonomic dysfunction.
Methods
Review of published papers indexed in medline/embase.
Results
Autonomic dysfunction has been reported in retrovirus (human immunodeficiency virus (HIV), human T-lymphotropic virus), herpes viruses, flavivirus, enterovirus 71 and lyssavirus infections. Autonomic dysfunction is relatively common in HIV-infected patients and heart rate variability is reduced even in early stages of infection. Orthostatic hypotension, urinary dysfunction and hypohidrosis have been described in tropical spastic paraparesis patients. Varicella zoster reactivation from autonomic ganglia may be involved in visceral disease and chronic intestinal pseudo-obstruction. Autonomic and peripheral nervous system dysfunction may happen in acute tick-borne encephalitis virus infections. Hydrophobia, hypersalivation, dyspnea, photophobia, and piloerection are frequently observed in human rabies. Autonomic dysfunction and vagal denervation is common in Chagas disease. Neuronal depopulation occurs mainly in chagasic heart disease and myenteric plexus, and megacolon, megaesophagus and cardiomyopathy are common complications in the chronic stage of Chagas disease. Parasympathetic autonomic dysfunction precedes left ventricle systolic dysfunction in Chagas disease. A high prevalence of subclinical autonomic neuropathy in leprosy patients has been reported, and autonomic nerve dysfunction may be an early manifestation of the disease. Autonomic dysfunction features in leprosy include anhidrosis, impaired sweating function, localised alopecia ,and reduced heart rate variability. Urinary retention and intestinal pseudo-obstruction have been described in Lyme disease. Diphtheritic polyneuropathy, tetanus and botulism are examples of bacterial infections releasing toxins that affect the autonomic nervous system.
Conclusions
Autonomic dysfunction may be responsible for additional morbidity in some infectious diseases.


Similar content being viewed by others
References
Brizzi KT, Lyons JL (2014) Peripheral nervous system manifestations of infectious diseases. Neurohospitalist 4:230–240
Carod-Artal (2016) Clinical management of infectious cerebral vasculitides. Expert Rev Neurother 16:205–221
Chow D, Nakamoto BK, Sullivan K, Sletten DM, Fujii S, Umekawa S et al (2015) Symptoms of autonomic dysfunction in human immunodeficiency virus. Open Forum Infect Dis. doi:10.1093/ofid/ofv103
Compostella C, Compostella L, D’Elia R (2008) The symptoms of autonomic dysfunction in HIV-positive Africans. Clin Auton Res 18:6
Robinson-Papp J, Sharma SK (2013) Autonomic neuropathy in HIV is unrecognized and associated with medical morbidity. AIDS Patient Care STD 27:539–543
Mittal CM, Wig N, Mishra S, Deepak KK (2004) Heart rate variability in human immunodeficiency virus-positive individuals. Int J Cardiol 94:1–6
Becker K, Gorlach I, Frieling T, Haussinger D (1997) Characterization and natural course of cardiac autonomic nervous dysfunction in HIV-infected patients. AIDS 11:751–757
Griffin GE, Miller A, Batman P, Forster SM, Pinching AJ, Harris JR et al (1998) Damage to jejuna intrinsic autonomic nerves in HIV infection. AIDS 2:379–382
Carod Artal FJ (2014) Infections of the spinal cord. In: Garcia Monco JC (ed) CNS infections, 1st edn. Springer, London, pp 181–210
Carod-Artal FJ, Mesquita HM, da Ribeiro LS (2008) Neurological symptoms and disability in HTLV-1 associated myelopathy. Neurologia 23:78–84
Caskey MF, Morgan DJ, Porto AF, Giozza SP, Muniz AL, Orge GO et al (2007) Clinical manifestations associated with HTLV type I infection: a cross-sectional study. AIDS Res Hum Retrovir 23:365–371
Alamy AH, Menezes FB, Leite AC, Nascimento OM, Araujo AQ (2001) Dysautonomia in human T-cell lymphotrophic virus type 1-associated myelopathy/tropical spastic paraparesis. Ann Neur 50:681–685
Ohishi K, Nagasato K, Aoi W, Nakamura T, Ichinose K, Nishiura Y et al (1993) Circadian rhythms of blood pressure and heart rate in patients with human T-lymphotrophic virus type-1-associated myelopathy. Tohoku J Exp Med 169:67–75
Raza SM, Pyatt JR (2006) Nocturnal hypertension and autonomic dysfunction due to human T-lymphotropic virus type-1 (HTLV-1)-associated myelopathy/tropical spastic paraparesis (HAM/TSP). Int J Cardiol 107:424–426
Kuriyama N, Niwa F, Watanabe Y, Yamada K, Tokuda T, Mizuno T et al (2009) Evaluation of autonomic malfunction in HTLV-1 associated myelopathy (HAM). Auton Neurosci 150:131–135
Gilden DH, Vafai A, Shtram Y, Becker Y, Devlin M, Wellish M (1983) Varicella-zoster virus DNA in human sensory ganglia. Nature 306:478–480
Nagel MA, Rempel A, Huntington J, Kim F, Choe A, Gilden D (2014) Frequency and abundance of alphaherpesvirus DNA in human thoracic sympathetic ganglia. J Virol 88:8189–8192
Gilden DH, Gesser R, Smith J, Wellish M, Laguardia JJ, Cohrs RJ et al (2001) Presence of VZV and HSV-1 DNA in human nodose and celiac ganglia. Virus Genes 23:145–147
Gilden D, Cohrs RJ, Mahalingam R, Nagel MA (2010) Neurological disease produced by varicella zoster virus reactivation without rash. Curr Top Microbiol Immunol 342:243–253
Khoury-Hanold W, Yordy B, Kong P, Kong Y, Ge W, Szigeti-Buck K et al (2016) Viral spread to enteric neurons links genital HSV-1 infection to toxic megacolon and lethality. Cell Host Microbe 19:788–799
Ives AM, Bertke AS (2017) Stress hormones epinephrine and corticosterone selectively modulate Herpes Simplex Virus 1 (HSV-1) and HSV-2 productive infections in adult sympathetic, but not sensory, neurons. J Virol 91(13). pii: e00582-17. doi:10.1128/JVI.00582-17
Brun P, Giron MC, Zoppellaro C, Bin A, Porzionato A, De Caro R et al (2010) Herpes simplex virus type 1 infection of the rat enteric nervous system evokes small-bowel neuromuscular abnormalities. Gastroenterology 138:1790–1801
Zoppellaro C, Bin A, Brun P, Banzato S, Macchi V, Castagliuolo I et al (2013) Adenosine-mediated enteric neuromuscular function is affected during herpes simplex virus type 1 infection of rat enteric nervous system. PLoS ONE 8:e72648
Chen JJ, Gershon AA, Li Z, Cowles RA, Gershon MD (2011) Varicella zoster virus (VZV) infects and establishes latency in enteric neurons. J Neurovirol 17:578–589
Carod Artal FJ (2017) The enteric nervous system: another forgotten autonomic target in viral infections? Clin Auton Res 27:137–138
Holland-Cunz S, Göppl M, Rauch U, Bär C, Klotz M, Schäfer KH (2006) Acquired intestinal aganglionosis after a lytic infection with varicella-zoster virus. J Pediatr Surg 41:e29–e31
Bennett JL, Mahalingam R, Wellish MC, Gilden DH (1996) Epstein-Barr virus-associated acute autonomic neuropathy. Ann Neurol 40:453–455
Dodig D, Ngo M, Bailey D, Bril V (2010) Brachial plexopathy complicating Epstein-Barr virus infection in an adult. Acta Myol 29:357–359
Ejima M, Ota K, Yamamoto K, Sugishita Y, Maruyama S (1994) A case of acute pandysautonomia and diffuse brain stem impairment associated with EB virus infection. Rinsho Shinkeigaku 34:1136–1141
Itoh Y, Oishi T, Ohnishi A, Murai Y, Imawatari R (1993) Acute cerebellar ataxia with sympathotonic orthostatic hypotension following Epstein-Barr virus infection–a case report. Rinsho Shinkeigaku 33:503–506
Besnard M, Faure C, Fromont-Hankard G, Ansart-Pirenne H, Peuchmaur M, Cezard JP et al (2000) Intestinal pseudo-obstruction and acute pandysautonomia associated with Epstein-Barr virus infection. Am J Gastroenterol 95:280–284
Uchida Y, Koike H, Oguri T, Kato H, Yuasa H, Mitake S (2015) Successful corticosteroid therapies in a case of acute motor, sensory, autonomic neuropathy after cytomegalovirus infection. Rinsho Shinkeigaku 55:339–344
Nakao K, Namekawa M, Kondo S, Ono S, Nakano I (2016) Subacute autonomic and sensory neuropathy closely related to cytomegalovirus infection preceded by frequent syncopal attacks. Rinsho Shinkeigaku 56:555–559
Neumann B, Schulte-Mattler W, Brix S, Pöschl P, Jilg W, Bogdahn U et al (2016) Autonomic and peripheral nervous system function in acute tick-borne encephalitis. Brain Behav 6:e00485
Kulakowska A, Byfield FJ, Zendzian-Piotrowska M, Zajkowska JM, Drozdowski W, Mrocko B et al (2014) Increased levels of sphingosine 1-phosphate in cerebrospinal fluid of patients diagnosed with tick-borne encephalitis. J Neuroinflamm 11:193
Kleiter I, Steinbrecher A, Flugel D, Bogdahn U, Schulte-Mattler W (2006) Autonomic involvement in tick-borne encephalitis (TBE): report of five cases. Eur J Med Res 11:61–265
Kaiser R (1999) The clinical and epidemiological profile of tick-borne encephalitis in southern Germany 1994–8. A prospective study of 656 patients. Brain 122:2067–2078
Nagata N, Iwata-Yoshikawa N, Hayasaka D, Sato Y, Kojima A, Kariwa H et al (2015) The pathogenesis of 3 neurotropic flaviviruses in a mouse model depends on the route of neuroinvasion after viremia. J Neuropathol Exp Neurol 74:250–260
Leis AA, Stokic DS (2012) Neuromuscular manifestations of West Nile virus infection. Front Neurol 3:37. doi:10.3389/fneur.2012.00037
Bode AV, Sejvar JJ, Pape WJ, Campbell GL, Marfin AA (2006) West Nile virus disease: a descriptive study of 228 patients hospitalized in a 4-county region of Colorado in 2003. Clin Infect Dis 42:1234–1240
Fratkin JD, Leis AA, Stokic DS, Slavinski SA, Geiss RW (2004) Spinal cord neuropathology in human West Nile virus infection. Arch Pathol Lab Med 128:533–537
Pepperell C, Rau N, Krajden S, Kern R, Humar A, Mederski B et al (2003) West Nile virus infection in 2002: morbidity and mortality among patients admitted to hospital in south central Ontario. CMAJ 168:1399–1405
Wang H, Siddharthan V, Hall JO, Morrey JD (2011) Autonomic nervous dysfunction in hamsters infected with West Nile virus. PLoS ONE 6:e19575
Wang H, Siddharthan V, Hall JO, Morrey JD (2014) Autonomic deficit not the cause of death in West Nile virus neurological disease. Clin Auton Res 24:15–23
Susilawathi NM, Darwinata AE, Dwija IB, Budayanti NS, Wirasandhi GA, Subrata K et al (2012) Epidemiological and clinical features of human rabies cases in Bali 2008–2010. BMC Infect Dis 12:81
Hemachudha T, Ugolini G, Wacharapluesadee S, Sungkarat W, Shuangshoti S, Laothamatas J (2013) Human rabies: neuropathogenesis, diagnosis, and management. Lancet Neurol 12:498–513
Pathak S, Horton DL, Lucas S, Brown D, Quaderi S, Polhill S et al (2014) Diagnosis, management and post-mortem findings of a human case of rabies imported into the United Kingdom from India: a case report. Virol J 11:63
Thong WY, Han A, Wang SJ, Lin J, Isa MS, Koay ES et al (2016) Enterovirus infections in Singaporean children: an assessment of neurological manifestations and clinical outcomes. Singap Med J. doi:10.11622/smedj.2016099
Selgrad M, De Giorgio R, Fini L, Cogliandro RF, Williams S, Stanghelini V et al (2009) JC virus infects the enteric glia of patients with chronic idiopathic intestinal pseudo-obstruction. Gut 58:25–32
Carod-Artal FJ, Gascon J (2010) Chagas disease and stroke. Lancet Neurol 9:533–542
Carod-Artal FJ (2013) Policy Implications of the changing epidemiology of Chagas disease and stroke. Stroke 44:2356–2360
Souza DH, Vaz Mda G, Fonseca CR, Luquetti A, Rezende Filho J, Oliveira EC (2013) Current epidemiological profile of Chagasic megaesophagus in Central Brazil. Rev Soc Bras Med Trop 46:316–321
Marin-Neto JA, Cunha-Neto E, Maciel BC, Simões MV (2007) Pathogenesis of chronic Chagas heart disease. Circulation 115:1109–1123
Pinazo MJ, Cañas E, Elizalde JI, García M, Gascón J, Gimeno F et al (2010) Diagnosis, management and treatment of chronic Chagas’ gastrointestinal disease in areas where Trypanosoma cruzi infection is not endemic. Gastroenterol Hepatol 33:191–200
Jabari S, de Oliveira EC, Brehmer A, da Silveira AB (2014) Chagasic megacolon: enteric neurons and related structures. Histochem Cell Biol 142:235–244
Carod-Artal FJ, Vargas AP, Horan TA, Nunes LG (2005) Chagasic cardiomyopathy is independently associated with ischemic stroke in Chagas disease. Stroke 36:965–970
Koberle F (1968) Chagas’ heart disease and Chagas’ syndromes: the pathology of American trypanosomiasis. Adv Parasitol 6:63–116
Mott KE, Hagstrom JW (1965) The pathologic lesions of the cardiac autonomic nervous system in chronic Chagas’ myocarditis. Circulation 31:273–286
Rassi A Jr, Neto Marin JA, Rassi A (2017) Chronic Chagas cardiomyopathy: a review of the main pathogenic mechanisms and the efficacy of aetiological treatment following the BENznidazole Evaluation for Interrupting Trypanosomiasis (BENEFIT) trial. Mem Inst Oswaldo Cruz 112:224–235
Junqueira LF Jr (2012) Insights into the clinical and functional significance of cardiac autonomic dysfunction in Chagas disease. Rev Soc Bras Med Trop 45:243–252
Costa HS, Nunes MC, Souza AC, Lima MM, Carneiro RB, Sousa GR et al (2015) Exercise-induced ventricular arrhythmias and vagal dysfunction in Chagas disease patients with no apparent cardiac involvement. Rev Soc Bras Med Trop 8:175–180
Vasconcelos DF, Junqueira LF Jr (2012) Cardiac autonomic and ventricular mechanical functions in asymptomatic chronic chagasic cardiomyopathy. Arq Bras Cardiol 98:111–119
Simões MV, Pintya AO, Sarabanda AV, Pazin-Filho A, Maciel BC, Marin-Neto JA (1999) Reduced [123I]MIBG uptake precedes wall motion impairment in Chagas’ disease. J Nucl Cardiol 6:S63
Ribeiro AL, Campos MS, Baptista LM, de Sousa MR (2010) The Valsalva maneuver in Chagas disease patients without cardiopathy. Clin Auton Res 20:79–83
Bowman NM, Kawai V, Gilman RH, Bocangel C, Galdos-Cardenas G, Cabrera L et al (2011) Autonomic dysfunction and risk factors associated with Trypanosome cruzi infection among children in Arequipa, Peru. Am J Trop Med Hyg 84:85–90
Thiers CA, Barbosa JL, Pereira Bde B, Nascimento EM, Nascimento JH, Medei EH et al (2012) Autonomic dysfunction and anti-M2 and anti-β1 receptor antibodies in Chagas disease patients. Arq Bras Cardiol 99:732–739
Barbosa-Ferreira JM, Mady C, Ianni BM, Lopes HF, Ramires FJ, Salemi VM et al (2015) Dysregulation of autonomic nervous system in Chagas’ heart disease is associated with altered adipocytokines levels. PLoS ONE 10:e0131447
World health Organization (2016) Global leprosy update, 2015: time for action, accountability and inclusion. Wkly Epidemiol Rec 35:405–420
Nascimento OJ (2013) Leprosy neuropathy: clinical presentations. Arq Neuropsiquiatr 71:661–666
Shetty VP, Mehta NH, Antia NH, Irani PF (1997) Teased fibre study of early nerve lesions in leprosy and contacts, with electrophysiological correlates. J Neurol Neurosurg Psychiatry 40:708
Soysal A, Atay T, Ozu T, Arpaci B (2004) Electrophysiological evaluation of peripheral and autonomic involvement in leprosy. Can J Neurol Sci 31:357–362
Illarramendi X, Bührer-Sékula S, Sales AM, Bakker MI, Oliveira A, Nery JA et al (2005) High prevalence of vasomotor reflex impairment in newly diagnosed leprosy patients. Eur J Clin Invest 35:658–665
Ulvi H, Yoldaş T, Yiğiter R, Müngen B (2003) R-R interval variation and the sympathetic skin response in the assessment of the autonomic nervous system in leprosy patients. Acta Neurol Scand 107:42–49
Vital RT, Illarramendi X, Nascimento O, Hacker MA, Sarno EN, Jardim MR (2012) Progression of leprosy neuropathy: a case series study. Brain Behav 2:249–255
Anand V, Pradhan S, Kumar P (2016) Autonomic neuropathy impairing quality of life after complication of MDT: are we managing enough? Lepr Rev 87:239–242
Yigiter R, Ulvi H (2011) Using the head-up tilt test as measured by EMG to assess autonomic nervous system involvement in people with leprosy. Clin Exp Med Lett 52:41–44
Halperin JJ, Volkman DJ, Wu P (1991) Central nervous system abnormalities in Lyme neuroborreliosis. Neurology 41:1571–1582
Shamim EA, Shamim SA, Liss G, Nylen E, Pincus JH, Yepes M (2005) Constipation heralding neuroborreliosis: an atypical tale of 2 patients. Arch Neurol 62:671–673
Chatila R, Kapadia CR (1998) Intestinal pseudoobstruction in acute Lyme disease: a case report. Am J Gastroenterol 93:1179–1180
Schefte DF, Nordentoft T (2015) Intestinal pseudoobstruction caused by chronic Lyme neuroborreliosis. A case report. J Neurogastroentgerol Motil 21:440–442
Puri BK, Shah M, Julu PO, Kingston MC, Monro JA (2013) Urinary bladder detrusor dysfunction symptoms in lyme disease. Int Neurourol J. 17:127–129
Olivares JP, Pallas F, Ceccaldi M et al (1995) Lyme disease presenting as isolated acute urinary retention caused by transverse myelitis: an electrophysiological and urodynamical study. Arch Phys Med Rehabil 76:1171–1172
Duray PH (1989) Histopathology of clinical phases of human Lyme disease. Rheum Dis Clin N Am 15:691–710
Noyes AM, Kluger J (2015) A tale of two syndromes: Lyme disease preceding postural orthostatic tachycardia syndrome. Ann Noninvasive Electrocardiol 20:82–86
Sibanc B, Lesnicar G (2002) Complex regional pain syndrome and Lyme borreliosis: two different diseases? Infection 6:396–399
Gila L, Guerrero A, Astarloa R, Martí P, Gutiérrez JM (1990) Reflex sympathetic dystrophy. A new manifestation of Lyme disease? Enferm Infecc Microbiol Clin 8:32–35
Bruckbauer HR, Preac Mursic V, Herzer P, Hofmann H (1997) Sudeck’s atrophy in Lyme borreliosis. Infection 25:372–376
Neumann RA, Aberer E, Stanek G (1989) Evidence for spirochetal origin of Sudeck’s atrophy (algodistrophy, reflex sympathetic dystrophy). Arch Orthop Surg 108:314–316
Sanghi V (2014) Neurologic manifestations of diphtheria and pertussis. Handb Clin Neurol 121:1355–1359
Mateen FJ, Bahl S, Khera A, Sutter RW (2013) Detection of diphtheritic polyneuropathy by acute flaccid paralysis surveillance, India. Emerg Infect Dis 19:1368–1373
Piradov MA, Pirogov VN, Popova LM, Avdunina IA (2001) Diphtheritic polyneuropathy: clinical analysis of severe forms. Arch Neurol 58:1438–1442
Logina I, Donaghy M (1999) Diphtheritic polyneuropathy: a clinical study and comparison with Guillain–Barré syndrome. J Neurol Neurosurg Psychiatry 67:433–438
Manikyamba D, Satyavani A, Deepa P (2015) Diphtheritic polyneuropathy in the wake of resurgence of diphtheria. J Pediatr Neurosci 10:331–334
Idiaquez J (1992) Autonomic dysfunction in diphtheritic neuropathy. J Neurol Neurosurg Psychiatry 55:159–161
Solders G, Nennesmo I, Persson A (1989) Diphtheritic neuropathy, an analysis based on muscle and nerve biopsy and repeated neurophysiological and autonomic function tests. J Neurol Neurosurg Psychiatry 52:876–880
Fisher CM, Adams RD (1956) Diphtheritic polyneuritis—a pathological study. J Neuropathol Exp Neurol 15:243
McAuley JH, Fearnley J, Laurence A, Ball JA (1999) Diphtheritic polyneuropathy. J Neurol Neurosurg Psychiatry 67:825–826
Freshwater-Turner D, Udy A, Lipman J, Deans R, Stuart J, Boots R et al (2007) Autonomic dysfunction in tetanus—what lessons can be learnt with specific reference to alpha-2 agonists? Anaesthesia 62:1066–1070
Lin TS, Chen LK, Lin TY, Wen SH, Chen MC, Jan RH (2011) Autonomic dysfunction because of severe tetanus in an unvaccinated child. Pediatr Neonatol 52:169–171
Wakerley BR, Yuki N (2015) Mimics and chameleons in Guillain–Barre and Miller Fisher syndromes. Pract Neurol 15:90–99
Topakian R, Heibl C, Stieglbauer K, Dreer B, Nagl M, Knoflach P et al (2009) Quantitative autonomic testing in the management of botulism. J Neurol 256:803–809
Merz B, Bigalke H, Stoll G, Naumann M (2003) Botulism type B presenting as pure autonomic dysfunction. Clin Auton Res 5:337–338
Patural H, Goffaux P, Paricio C, Emeriaud G, Teyssier G, Barthelemy JC et al (2009) Infant botulism intoxication and autonomic nervous system dysfunction. Anaerobe 15:197–200
Potulska-Chromik A, Zakrzewska-Pniewska B, Szmidt-Salkowska E, Lewandowski J, Sinski M, Pryjalkowski W et al (2013) Long lasting dysautonomia due to botulinum toxin B poisoning: clinical-laboratory follow up and difficulties in initial diagnosis. BMC Res Notes 6:438
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The author have not conflicts of interest about this article.
Rights and permissions
About this article
Cite this article
Carod-Artal, F.J. Infectious diseases causing autonomic dysfunction. Clin Auton Res 28, 67–81 (2018). https://doi.org/10.1007/s10286-017-0452-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10286-017-0452-4