Abstract
The field of immunology is fundamental to our understanding of the intricate dynamics of the tumor microenvironment. In particular, tumor-infiltrating lymphocyte (TIL) assessment emerges as essential aspect in breast cancer cases. To gain comprehensive insights, the quantification of TILs through computer-assisted pathology (CAP) tools has become a prominent approach, employing advanced artificial intelligence models based on deep learning techniques. The successful recognition of TILs requires the models to be trained, a process that demands access to annotated datasets. Unfortunately, this task is hampered not only by the scarcity of such datasets, but also by the time-consuming nature of the annotation phase required to create them. Our review endeavors to examine publicly accessible datasets pertaining to the TIL domain and thereby become a valuable resource for the TIL community. The overall aim of the present review is thus to make it easier to train and validate current and upcoming CAP tools for TIL assessment by inspecting and evaluating existing publicly available online datasets.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Breast cancer (BC) is the most common cancer in women and is a significant threat to public health worldwide [1]. The role of immune cells in the primary tumor in BC disease progression cannot be underestimated since, by modeling the immunological context, they reflect the immune response determined at the time of diagnosis [2, 3]. The ability to estimate the prognostic value of immune cells has been refined further with the rise of more reliable methods to determine immune cell functional status and phenotypes. The findings demonstrate that immune cells in BC can affect cancer cell behavior and that the malignant disease arises [4], in part, by a bilateral relation between cancer cells and their immunological microenvironment [5,6,7,8].
Lymphocytes have been identified as one of the immune cells with the strongest prognostic implications of the various forms of immune populations [9]. Higher concentrations of tumor-infiltrating lymphocytes (TILs), lymphocytes that migrate into the tumor microenvironment, have been associated with better prognosis and treatment response in BC, especially in two subtypes: HER2-positive and triple-negative breast cancer (TNBC) [10, 11].
An initial finding of Aaltomaa et al. has prompted significant and ongoing TIL assessment and research [12, 13], leading to the establishment of the TIL Working Group (TIL-WG), an international group of experts whose main objective is to update TIL assessment guidelines and, in particular, to standardize Visual TIL Assessment (VTA) [14]. The TIL-WG strives to minimize the subjectivity in evaluation that arises from the high level of inter-observer variation between specialists [2, 15,16,17].
By studying the spatial distribution and quantity of TILs, assessed by a histopathological examination using hematoxylin and eosin (H&E)-stained images [2], experts can gain valuable insights into patients’ prognosis and response to treatment [18].
For a comprehensive understanding of the immune landscape of BC, we can also take into consideration immunohistochemistry (IHC)-stained images of biomarkers related to TILs, like CD4, CD8, FOXP3, CD20, and CD22 biomarkers [19,20,21,22,23]. The first three of these highlight some subclasses of a type of immune cell, the T lymphocyte, that appears in the presence of infected or cancerous cells [24]. The latter two highlight B lymphocytes [25], which are also evaluated as TILs in H&E-stained images. Note that only H&E-stained images are used to evaluate TILs in clinical practice, whereas IHC-stained images are still used for research purposes only [2].
The evaluation of biomarkers related to TILs in H&E or IHC-stained images has shown significant potential when based on advanced artificial intelligence (AI) techniques, such as deep learning (DL) [26,27,28]. These methods have the potential to yield promising results that overcome the problem of inter-observer variation, but more data and more parameters need to be incorporated to create more accurate and powerful models.
DL-based approaches in BC research should be trustworthy and reliable, so efforts should be made to collect a variety of representative datasets that are free from bias and to verify the consistency of the annotations within the datasets to minimize errors made during the annotation process by introducing data quality-control procedures. An example of such a procedure is the use of relations verification defined in a spatial logic by applying Discrete Mereotopology techniques to Mathematical Morphology [29, 30].
To reinforce the value of TILs, we will first address the importance of the immune response, which plays a significant part in cancer evolution. We will describe the assessment of TILs using histological image analysis employing digital pathology, a promising and growing area of research in the field of immune responses. We will then discuss the development of computer-assisted pathology (CAP) tools, which require massive amounts of data to train models using techniques involving advanced AI algorithms. In addition, we will suggest ways to make the TIL annotation process less time-consuming in future datasets, while maintaining its ground-truthness. At this point, we will highlight the primary aim of our review, which is to provide a perspective on the datasets used for TIL assessment, in particular those of publicly available histological images, to see whether the TIL research works with the same batch of data. To broaden the spectrum of the datasets and to allow the models to be more widely generalizable, we will also look for more available data on TILs in other tumors. For each dataset, we will then evaluate the main approaches for assessing TILs based on the types of annotations provided. Thereafter, we will discuss the ideas about the datasets that could contribute most substantively to the research by filling in gaps or addressing limitations evident in the current literature.
TIL Assessment for Evaluating Cancer Progression
TILs are predictive prognostic markers in BC because they provide a snapshot of the tumor scenario and are one of the best examples of the association between natural defenses and carcinogenesis [31]. Thus, we can conceive of TILs as an unloaded weapon whose drug-induced reactivation can lead to the restoration of formerly fully operational natural anti-cancer defenses [31].
TILs are essential for analyzing the immunological environment of BC and other malignancies like colorectal cancer or other kinds of solid tumors [17, 32, 33].
TILs should be frequently evaluated as novel prognostic and therapy-predicting markers, particularly in the most aggressive breast lesions, such as the triple-negative and HER2-positive molecular subvariants [31, 34]. TILs have been studied independently of immune blockade agents as prognostic indicators influencing BC outcomes in chemotherapy trials in several publications [31, 35, 36].
However, TIL assessment has not featured in pathological reports even when it is accepted as a prognostic factor, as stated in the St. Gallen 2023 guidelines [37]. The role of TILs in treatment decisions remains unclear because the data on TILs are still considered insufficient to enable a reliable choice of specific therapy regimens to be made and to decide whether to withhold treatment [31, 38]. As a result, currently clinicians are not recommended to base their therapy decisions solely on TILs [38]. For this reason, TILs should not be treated as an independent variable [39, 40], but rather interpreted in conjunction with other prognostic variables like tumor and lymph node status to provide clinicians with all the prognostic information they need to examine treatment options reliably with their patients [38].
In the near future, TIL research will be able to guarantee a novel standardization of TIL assessment by improving on the approximate semiquantitative evaluation that is currently practiced, which is affected by a substantial degree of inter-observer variation [2, 14].
Enhancing Diagnosis Through Computer-Assisted Pathology
One of the primary advantages of digital slides over traditional glass slides is the ability to apply quantitative automatic image analysis algorithms with the introduction of AI techniques, leading to the creation of computer-assisted pathology (CAP) tools [41].
By using these instruments, it is possible to reduce inter-observer error and subjectivity of pathologists and thereby help them with the assessment process [14].
CAP tools, with AI integration, offer support in a range of tasks related to computer vision; “Computer Vision Tasks in CAP Tools” section describes possible applications. However, to fully exploit the power of AI in CAP tools, large-scale annotated datasets are indispensable. Given the time-consuming nature of dataset creation, in “Optimize the Annotation Phase Time” section, we will delve into methods to expedite and enhance this process.
Computer Vision Tasks in CAP Tools
Given the advances in computer vision and AI, CAP tools are becoming increasingly important in digital pathology tasks [41], such as automatic tissue segmentation and nucleus detection [42,43,44]. Even though certain CAP tools can quantify specific nuclei, such as TILs, they do so with varying degrees of difficultly. By considering TILs, a classification strategy can determine whether there are TILs present in a given image. By employing the localization approach, it is possible to specify the regions where TILs are located, like box shapes [45].
This strategy can be beneficial to weakly supervised learning, in which a whole slide image (WSI) that we claim contains TILs can be split into tiles and the locations of the TILs then checked [46]. By so doing, we can gain an approximate idea of where the TILs are in terms of spatial localization, even if this is not sufficient to allow them to be quantified [47].
Another helpful method for quantification that is more complex than combining classification and localization involves drawing a box around each TIL and counting the frequency of boxes [48, 49]. A variant of the method consists of placing a point over the object instead of a bounding box. This is useful when, for example, there are multiple small objects (as is the case in TIL detection), since under such circumstances box usage would cause too much confusion and, due to the overlap of many boxes, would give unclear results [50].
The next stage is semantic segmentation, which involves drawing a boundary around each object and determining the pixel-level features. Semantic segmentation entails labeling every pixel in an image and determining the class to which it belongs [51]. It is feasible to define which pixels are not part of a TIL in this manner [52, 53]. However, if there are more nearby TILs, we will not be able to estimate their frequency accurately, but instead, we will see a region with TILs [48]. For example, the segmentation mask task can be beneficial for identifying tissue regions, so objectives are not directly related to quantification. Nevertheless, it can be of practical value to distinguish between stromal and intratumoral TILs, which is essential for the obtaining a TIL score in BC [2, 14].
Finally, there is a process, known as instance segmentation, that advances semantic segmentation. Rather than giving all objects in a class equal pixel values, this process aims to segment and display various instances of the same class [54]. By doing so, we may establish more exact boundaries for semantic segmentation and object identification, by which means we can determine the number of objects [55,56,57].
Optimize the Annotation Phase Time
To effectively train CAP tools, it is essential to annotate WSIs. Pathologists typically begin this process by manually annotating a limited number of WSIs [58]. These annotations serve as the initial labeled data for CAP tool training [59, 60]. Once this first step has been completed, the remaining WSIs can be annotated using a semi-supervised learning approach, which reduces the amount of manual annotation by allowing pathologists to intervene only to ensure the accuracy and refinement of the generated annotations [61, 62].
With the advent of DL techniques, there has been an increase in demand for many annotations [61]. DL models have a considerable capacity to learn detailed patterns and features from data due to their complex architectures. While DL models require a large amount of training data, they have the potential to yield outstanding performance levels.
As a result, having a large dataset with numerous annotations becomes critical if the promise of DL in pathology is to be fully realized [63].
Collecting these data can be time-consuming, particularly when manual annotations, which can also become monotonous and repetitive, are involved. For these reasons, there is an urgent need to identify novel techniques to improve the stage of dataset creation [58, 61, 64].
We may discover smart approaches to simplifying the process by carefully reviewing the annotation methods used in some of the datasets mentioned hereafter.
First, we note that a common approach to achieving large numbers of annotations consists simply of having more people making the annotations. Since it is difficult to find several expert pathologists who are available for the task, structured crowdsourcing is a possible approach, whereby people with less expertise make annotations in accordance with their level of skills, and their work is mentored and eventually corrected by expert pathologists [65, 66].
The review phase consists of correcting and giving an overlay of the segmentation. In [65], the review phase was mainly exploited for annotating non-predominant or challenging classes. Production of the latest version of this dataset, which is mentioned in the work of Amgad et al., involved an intriguing new technique that includes non-pathologist nucleus labels [64]. Two main approaches were employed in this work: one focused on breadth, gathering single-rater annotations over many fields of view (FOVs) to obtain the majority of the data in the study, while the other assessed interrater reliability and agreement by gathering annotations from numerous non-pathologists for a smaller selection of common FOVs. Pathologists also provided annotations for these FOVs to assess non-pathologists’ reliability [64].
To lower the labeling burden, the method of initial labeling followed by a review by professional pathologists is employed. However, ensuring the accuracy of labeling by non-pathologists remains a challenge. The re-examination process is still time-consuming and labor-intensive if the initial annotation is not of high quality, and it requires the involvement of multiple specialists to prevent subjective errors [64].
Amgad et al. also introduced an algorithmic recommendation for nucleus boundaries and classes that provides instructions to annotate other nuclei with bounding boxes by clicking on nuclei with correct border recommendations [64].
Adding an automatic proposal, made, for instance, by a DL model, provides an iterative learning strategy whereby each iteration produces better annotation suggestions that require less manual adjustment [61, 67].
We suggest further approaches to the introduction of annotations by considering different people with variable levels of expertise, allowing non-experts to carry out the main tasks at their level of skill under the supervision of expert pathologists. Tools like MONAI [68, 69] and Quick Annotator [58] are available to make this manual adjustment and thereby facilitate better automatic annotation proposals. These tools, which use an active learning framework for continuous learning, can be integrated into digital pathology and WSI analysis platforms like QuPath [70].
Using these tools makes it possible to take advantage of the efficiency of weak labeling methods, which need substantially less time and resources. We can extract further annotations from unlabeled images by starting with a small set of annotations made by domain experts [47]. This idea of generating annotations from unlabeled data was previously investigated in traditional CAP tools, which used image-processing-based approaches like thresholding to extract annotations [71]. These rule-based techniques, however, are extremely task-specific and require domain expertise for troubleshooting and optimization.
Comparative Analysis of Datasets for TIL Research
This section aims to provide an overview of the publicly available datasets for TIL assessment using H&E images (Table 1). On Table 1, detailed information such as magnifications and size of the datasets is presented, enhancing our understanding of their composition and potential usability.
For a more complete perspective of the available datasets, we extend the search to encompass cancer types in addition to BC. We also examine datasets not based on TILs but also those for lymphocytes and inflammatory cells. TILs are specific kinds of lymphocytes, so we can gather additional information from datasets for lymphocyte evaluation, such as that on morphological features, for TIL assessment [72]. We then look for datasets that assess inflammatory cells because these can be relevant for TIL scoring when performed on round inflammatory cells, omitting polymorphonuclear cells solely in the intratumoral region in cutaneous melanoma [73]. As a result, we can use inflammatory cell datasets from different cancer types to generalize predictive models [74].
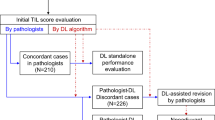
As can be seen in Table 1, some datasets are a combination, or a selection of some parts, of other datasets. Figure 1 outlines the dependencies of the datasets described in Table 1.
Dependencies in the TIL datasets described in Table 1. The years indicate when datasets were created; the arrows indicate the dependencies between them
We recognize that there are other online datasets, but access to them is either restricted or requires prior registration.
One important dataset that requires prior registration is the ATLAS of Histopathology database, a large-scale, patch-level annotation of different components of tissues created in 2019 by the Multimedia Lab of the University of Toronto. This dataset requires an End User License Agreement (EULA) to be accepted [93].
There are datasets for TIL assessment without annotation that feature only associated clinical data. One such example is that proposed by Shvetsov and coworkers, called UiT-TILs, that can be used to clinically validate TIL classifications [94]. The UiT-TILs dataset contains 1189 image patches from 87 non-small cell lung cancer (NSCLC) patients with matched clinical data, and it is a subset of another dataset, reported by Rakaee et al. [95]. A similar dataset was presented by Fassler’s team, in which they processed data not only from the TCGA, but also from the UNC CBCS Phase 3 cohort, which contains 2998 cases with 1138 diagnostic WSIs from representative blocks, and related follow-up recurrence and survival data [96, 97].
Dataset Goals Over the Years
In the first part of “Comparative Analysis of Datasets for TIL Research” section, we analyzed the datasets for evaluating TILs, lymphocytes and inflammatory cells. This raises questions about whether the focus of these datasets has changed over time and what subjects and issues they target. In search of answers, we extracted the keywords of each abstract through ChatGPT APIs since this is a fast and intuitive method. We decided not to examine author-entered keywords to minimize potential bias and ensure universality since some dataset articles do not include author-entered keywords.
First, we extracted the texts of the abstracts from the papers relating to the datasets shown in Table 1. We then applied OpenAI APIs to them in order to ask ChatGPT to identify the main words in each paper’s abstract. We did this because we assumed that most of the important keywords are almost always mentioned in a paper’s abstract. Finally, we grouped the keywords from the dataset articles by year and carried out topic modeling for each year using a Latent Dirichlet Allocation (LDA) model [98], which enabled us to discover the hidden relationships in the keyword collection and ultimately the main topic for each year [99, 100].
Figure 2 shows how the dataset goals change over time, as revealed by the analysis of the LDA model outcome. It is clear that the research community has continued to present ever-larger datasets over the years because the advent of DL has meant that we now need substantial quantities of data to train neural networks.
We note that the research community has invested significant resources and will continue to do so in order to make annotated datasets for training CAP tools. While these technologies use efficient and innovative approaches to save time, the process remains complex and demands effective communication among experts from diverse sectors [41].
The aforementioned trend shows that TIL evaluation is becoming more dependent on the contribution of CAP tools. This is crucial because it will progressively reduce the differences from expert assessments over time [14]. As a result, in the TIL evaluation scenario, we note a growing inclination to merge the experience of experts from different fields [2, 14].
Conclusions and Proposals for Future Challenges
This review compares datasets used for TIL assessment and encompasses datasets about lymphocytes and inflammatory cells, since, as mentioned in “Comparative Analysis of Datasets for TIL Research” section, they are related to the TIL assessment scenario.
The research community is working to make larger datasets for TIL assessment and should also provide novel TIL datasets for different tumor types in addition to BC to enhance this area of investigation [92]. It is essential to include annotated images from various scanners in order to leverage models trained on these datasets effectively. This approach ensures improved generalization of the TIL assessment, making the models helpful across a variety of scanners rather than being limited to the performance of a specific one [101,102,103].
By adopting this strategy, CAP tools will become more valuable to experts and enable them to carry out TIL assessments of which they can have greater confidence.
As stated in “Introduction” section, TIL assessment is achieved using H&E images. Nevertheless, there is a supplementary and non-standardized method for measuring TILs, consisting of quantifying immune biomarkers of specific subpopulations of TILs such as CD4, CD8, FOXP3, CD20, and CD22 [19,20,21,22,23]. The information about the immune markers can provide us with more insights into TILs and about their distribution and spatial relationships, as shown by two studies [104, 105]. However, we require IHC images of them, such as sections from the paraffin block, to make this evaluation. It should be noted that the sections, even if very close (e.g., 4 \(\mu\)m [106]) and from the same paraffin block, can vary slightly [107]. There are also a large number of variables that influence antigen staining in paraffin-embedded tissues, such as the type of fixative, fixation time, tissue processing, the level of antigen expression and preservation, and also the clone and the dilution of the antibody used, the antigen-retrieval method, and the detection system and chromogen [108, 109]. Other procedures use multiplexed IHC images to apply diverse IHC staining in a single section. However, these are more expensive and can be beset with problems of antibody compatibility and tissue penetration [110].
To broaden the scope of TIL assessment research, the research community should make these IHC images public and widely available. By doing so, or, better yet, by offering the ground truth, we can rise to exciting challenges like the one overcome to distinguish HER2-positive from HER2-negative BC specimens solely through the evaluation of H&E slides [111]. Thus, a novel aim should be to quantify immune markers directly on H&E-stained images.
Data Availability
Public and available datasets supporting the findings of the review are available within the article.
Abbreviations
- AI:
-
Artificial Intelligence
- BC:
-
Breast cancer
- API:
-
Application programming interface
- CAP:
-
Computer-assisted pathology
- CC:
-
Creative Commons
- Curie:
-
Institut Curie
- CWRUC:
-
Case Western Reserve University
- DL:
-
Deep learning
- DS1/DS2:
-
Dataset 1/Dataset 2
- EULA:
-
End User License Agreement
- FOV:
-
Field of view
- GBM:
-
Glioblastoma multiforme tumor
- H&E:
-
Hematoxylin & eosin
- HER2:
-
Human epidermal growth factor receptor 2
- HNSCC:
-
Head and neck squamous cell carcinoma
- IHC:
-
Immunohistochemistry
- IITG:
-
Indian Institute of Technology Guwahati
- JB:
-
Jules Bordet Institute
- LDA:
-
Latent Dirichlet Allocation
- LGG:
-
Lower grade glioma tumor
- NSLC:
-
Non-small cell lung cancer
- ROI:
-
Region of interest
- RUMC:
-
Radboud University Medical Center
- SHSC:
-
Sunnybrook Health Sciences Centre
- sTIL:
-
Stromal tumor-infiltrating lymphocyte
- TCGA:
-
The Cancer Genome Atlas Program
- TCIA:
-
The Cancer Imaging Archive
- TIL:
-
Tumor-infiltrating lymphocyte
- TIL-WG:
-
TIL working group
- TNBC:
-
Triple-negative breast cancer
- UHCW:
-
University Hospitals Coventry and Warwickshire
- VTA:
-
Visual tumor-infiltrating lymphocyte assessment
- WSI:
-
Whole slide image
References
Siegel, R.L., Miller, K.D., Wagle, N.S., Jemal, A.: Cancer statistics, 2023. Ca Cancer J Clin 73(1), 17–48 (2023)
Salgado, R., Denkert, C., Demaria, S., Sirtaine, N.: The evaluation of tumor-infiltrating lymphocytes (TILs) in breast cancer: recommendations by an International TILs Working Group 2014. Elsevier (2015)
Galon, J., Dieu-Nosjean, M., Tartour, E., Sautes-Fridman, C., Fridman, W., et al: Immune infiltration in human tumors: a prognostic factor that should not be ignored. Oncogene 29(8), 1093–1102 (2010)
Hanahan, D., Weinberg, R.A.: Hallmarks of cancer: the next generation. cell 144(5), 646–674 (2011)
DeNardo, D.G., Andreu, P., Coussens, L.M.: Interactions between lymphocytes and myeloid cells regulate pro-versus anti-tumor immunity. Cancer and Metastasis Reviews 29, 309–316 (2010) https://doi.org/10.1007/S10555-010-9223-6
Fridman, W.H., Pagès, F., Sautès-Fridman, C., Galon, J.: The immune contexture in human tumours: impact on clinical outcome. Nature Reviews Cancer 12(4), 298–306 (2012)
Savas, P., Salgado, R., Denkert, C., Sotiriou, C., Darcy, P.K., Smyth, M.J., Loi, S.: Clinical relevance of host immunity in breast cancer: from TILs to the clinic. Nature reviews Clinical oncology 13(4), 228–241 (2016)
Soongsathitanon, J., Jamjuntra, P., Sumransub, N., Yangngam, S., Fuente, M., Landskron, G., Thuwajit, P., Hermoso, M.A., Thuwajit, C.: Crosstalk between tumor-infiltrating immune cells and cancer-associated fibroblasts in tumor growth and immunosuppression of breast cancer. Journal of Immunology Research 2021 (2021)
Burugu, S., Asleh-Aburaya, K., Nielsen, T.: Immune infiltrates in the breast cancer microenvironment: detection, characterization and clinical implication. Breast Cancer 24 (2016) https://doi.org/10.1007/s12282-016-0698-z
Adams, S., Gray, R.J., Demaria, S., Goldstein, L., Perez, E.A., Shulman, L.N., Martino, S., Wang, M., Jones, V.E., Saphner, T.J., Wolff, A.C., Wood, W.C., Davidson, N.E., Sledge, G.W., Sparano, J.A., Badve, S.S.: Prognostic value of tumor-infiltrating lymphocytes in triple-negative breast cancers from two phase iii randomized adjuvant breast cancer trials: Ecog 2197 and ecog. J Clin Oncol 32, 2959–2966 (2014) https://doi.org/10.1200/JCO.2013.55.0491
Denkert, C., Loibl, S., Noske, A., Roller, M., Müller, B., Komor, M., Budczies, J., Darb-Esfahani, S., Kronenwett, R., Hanusch, C., Törne, C., Weichert, W., Engels, K., Solbach, C., Schrader, I., Dietel, M.: Tumor-associated lymphocytes as an independent predictor of response to neoadjuvant chemotherapy in breast cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 28, 105–13 (2009) https://doi.org/10.1200/JCO.2009.23.7370
Aaltomaa, S., Lipponen, P., Eskelinen, M., Kosma, V.M., Marin, S., Alhava, E., nen, K.: Lymphocyte infiltrates as a prognostic variable in female breast cancer. Eur J Cancer 28A(4-5), 859–864 (1992)
Valenza, C., Taurelli Salimbeni, B., Santoro, C., Trapani, D., Antonarelli, G., Curigliano, G.: Tumor Infiltrating Lymphocytes across Breast Cancer Subtypes: Current Issues for Biomarker Assessment. Cancers 15(3), 767 (2023)
Amgad, M., Stovgaard, E., Balslev, E., Thagaard, J.: Report on computational assessment of tumor infiltrating lymphocytes from the international immuno-oncology biomarker working group. nature.com (2020)
Swisher, S.K., Wu, Y., Castaneda, C.A., Lyons, G.R., Yang, F., Tapia, C., Wang, X., Casavilca, S.A., Bassett, R., Castillo, M., et al: Interobserver agreement between pathologists assessing tumor-infiltrating lymphocytes (TILs) in breast cancer using methodology proposed by the International TILs Working Group. Annals of surgical oncology 23, 2242–2248 (2016)
Van Bockstal, M.R., François, A., Altinay, S., Arnould, L., Balkenhol, M., Broeckx, G., Burguès, O., Colpaert, C., Dedeurwaerdere, F., Dessauvagie, B., et al: Interobserver variability in the assessment of stromal tumor-infiltrating lymphocytes (sTILs) in triple-negative invasive breast carcinoma influences the association with pathological complete response: The IVITA study. Modern Pathology 34(12), 2130–2140 (2021)
Hendry, S., Salgado, R., Gevaert, T., Russell, P.A., John, T., Thapa, B., Christie, M., Van De Vijver, K., Estrada, M.V., Gonzalez-Ericsson, P.I., et al: Assessing tumor infiltrating lymphocytes in solid tumors: a practical review for pathologists and proposal for a standardized method from the International Immuno-Oncology Biomarkers Working Group: Part 2: TILs in melanoma, gastrointestinal tract carcinomas, non-small cell lung carcinoma and mesothelioma, endometrial and ovarian carcinomas, squamous cell carcinoma of the head and neck, genitourinary carcinomas, and primary brain tumors. Advances in anatomic pathology 24(6), 311 (2017)
El Bairi, K., Haynes, H.R., Blackley, E., Fineberg, S., Shear, J., Turner, S., De Freitas, J.R., Sur, D., Amendola, L.C., Gharib, M., et al: The tale of TILs in breast cancer: a report from the international immuno-oncology biomarker working group. NPJ Breast Cancer 7(1), 150 (2021)
Takenaka, M., Seki, N., Toh, U., Hattori, S., Kawahara, A., Yamaguchi, T., Koura, K., Takahashi, R., Otsuka, H., Takahashi, H., Iwakuma, N., Nakagawa, S., Fujii, T., Sasada, T., Yamaguchi, R., Yano, H., Shirouzu, K., Kage, M.: FOXP3 expression in tumor cells and tumor-infiltrating lymphocytes is associated with breast cancer prognosis. Molecular and clinical oncology 1, 625–632 (2013) 10.3892/mco.2013.107
Locy, H., Verhulst, S., Cools, W., Waelput, W., Brock, S., Cras, L., Schiettecatte, A., Jonckheere, J., Grunsven, L.A., Vanhoeij, M., et al: Assessing tumor-infiltrating lymphocytes in breast cancer: a proposal for combining immunohistochemistry and gene expression analysis to refine scoring. Frontiers in Immunology 13, 794175 (2022)
Huertas-Caro, C.A., Ramirez, M.A., Gonzalez-Torres, H.J., Sanabria-Salas, M.C., Serrano-Gomez, S.J.: Immune Lymphocyte Infiltrate and its Prognostic Value in Triple-Negative Breast Cancer. Frontiers in Oncology 12, 910976 (2022)
Chu, P.G., Loera, S., Huang, Q., Weiss, L.M.: Lineage determination of CD20–B-cell neoplasms: an immunohistochemical study. American journal of clinical pathology 126(4), 534–544 (2006)
Zaib, T., Cheng, K., Liu, T., Mei, R., Liu, Q., Zhou, X., He, L., Rashid, H., Xie, Q., Khan, H., et al: Expression of CD22 in Triple-Negative Breast Cancer: A Novel Prognostic Biomarker and Potential Target for CAR Therapy. International Journal of Molecular Sciences 24(3), 2152 (2023)
Carvalho, M.I., Pires, I., Prada, J., Queiroga, F.L., et al.: A role for T-lymphocytes in human breast cancer and in canine mammary tumors. BioMed Research International 2014 (2014)
Qin, Y., Peng, F., Ai, L., Mu, S., Li, Y., Yang, C., Hu, Y.: Tumor-infiltrating B cells as a favorable prognostic biomarker in breast cancer: a systematic review and meta-analysis. Cancer Cell International 21(1), 1–8 (2021)
Lu, Z., Xu, S., Shao, W., Wu, Y., Zhang, J., Han, Z., Feng, Q., Huang, K.: Deep-learning–based characterization of tumor-infiltrating lymphocytes in breast cancers from histopathology images and multiomics data. JCO clinical cancer informatics 4, 480–490 (2020)
Mi, H., Gong, C., Sulam, J., Fertig, E., Szalay, A., Jaffee, E., Stearns, V., Emens, L., Cimino-Mathews, A., Popel, A.: Digital Pathology Analysis Quantifies Spatial Heterogeneity of CD3, CD4, CD8, CD20, and FoxP3 Immune Markers in Triple-Negative Breast Cancer. Frontiers in Physiology 11 (2020) https://doi.org/10.3389/fphys.2020.583333
Balkenhol, M., Ciompi, F., Swiderska, Z., Loo, R., Intezar, M., Otte-Höller, I., Geijs, D., Lotz, J., Weiss, N., Bel, T., Litjens, G., Bult, P., Laak, J.: Optimized tumour infiltrating lymphocyte assessment for triple negative breast cancer prognostics. Breast 56, 78–87 (2021)
Randell, D.A., Landini, G., Galton, A.: Discrete Mereotopology for Spatial Reasoning in Automated Histological Image Analysis. IEEE Trans Pattern Anal Mach Intell 35(3), 568–581 (2013)
Galton, A.: Discrete Mereotopology, pp. 293–321 (2014). https://doi.org/10.1007/978-3-319-05356-1_11
Zgura, A., Galesa, L., Bratila, E., Anghel, R.: Relationship between tumor infiltrating lymphocytes and progression in breast cancer. Maedica 13(4), 317 (2018)
Bai, Z., Zhou, Y., Ye, Z., Xiong, J., Lan, H., Wang, F.: Tumor-infiltrating lymphocytes in colorectal cancer: the fundamental indication and application on immunotherapy. Frontiers in Immunology 12, 5926 (2022)
Hendry, S., Salgado, R., Gevaert, T., Russell, P.A., John, T., Thapa, B., Christie, M., Van De Vijver, K., Estrada, M.V., Gonzalez-Ericsson, P.I., et al: Assessing tumor infiltrating lymphocytes in solid tumors: A practical review for pathologists and proposal for a standardized method from the International Immuno-Oncology Biomarkers Working Group: Part 1: Assessing the host immune response, TILs in invasive breast carcinoma and ductal carcinoma in situ, metastatic tumor deposits and areas for further research. Advances in anatomic pathology 24(5), 235 (2017)
Stanton, S.E., Disis, M.L.: Clinical significance of tumor-infiltrating lymphocytes in breast cancer. Journal for immunotherapy of cancer 4, 1–7 (2016)
Cejuela, M., Vethencourt, A., Pernas, S.: Immune checkpoint inhibitors and novel immunotherapy approaches for breast cancer. Current Oncology Reports 24(12), 1801–1819 (2022)
Force, J., Leal, J.H.S., McArthur, H.L.: Checkpoint blockade strategies in the treatment of breast cancer: where we are and where we are heading. Current treatment options in oncology 20, 1–14 (2019)
Balic, M., Thomssen, C., Gnant, M., Harbeck, N.: St. Gallen/Vienna 2023: Optimization of Treatment for Patients with Primary Breast Cancer–A Brief Summary of the Consensus Discussion. Breast Care 18(3), 213–222 (2023)
Laenkholm, A.-V., Callagy, G., Balancin, M., Bartlett, J.M., Sotiriou, C., Marchio, C., Kok, M., Dos Anjos, C.H., Salgado, R.: Incorporation of TILs in daily breast cancer care: how much evidence can we bear? Virchows Archiv 480(1), 147–162 (2022)
Burstein, H.J., Curigliano, G., Loibl, S., Dubsky, P., Gnant, M., Poortmans, P., Colleoni, M., Denkert, C., Piccart-Gebhart, M., Regan, M., et al: Estimating the benefits of therapy for early-stage breast cancer: the St. Gallen International Consensus Guidelines for the primary therapy of early breast cancer 2019. Annals of Oncology 30(10), 1541–1557 (2019)
Cardoso, F., Kyriakides, S., Ohno, S., Penault-Llorca, F., Poortmans, P., Rubio, I.T., Zackrisson, S., Senkus, E.: Early breast cancer: Esmo clinical practice guidelines for diagnosis, treatment and follow-up. Annals of Oncology 30(8), 1194–1220 (2019) 10.1093/annonc/mdz173 . Triple-negative breast cancer - clinical results and biomarker analysis of GeparNuevo study
Nam, S., Chong, Y., Jung, C.K., Kwak, T.-Y., Lee, J.Y., Park, J., Rho, M.J., Go, H.: Introduction to digital pathology and computer-aided pathology. Journal of pathology and translational medicine 54(2), 125–134 (2020)
Abdolhoseini, M., Kluge, M.G., Walker, F.R., Johnson, S.J.: Segmentation of heavily clustered nuclei from histopathological images. Scientific reports 9(1), 4551 (2019)
Sun, M., Zou, W., Wang, Z., Wang, S., Sun, Z.: An Automated Framework for Histopathological Nucleus Segmentation with Deep Attention Integrated Networks. IEEE/ACM Transactions on Computational Biology and Bioinformatics (2023)
Salvi, M., Molinaro, L., Metovic, J., Patrono, D., Romagnoli, R., Papotti, M., Molinari, F.: Fully automated quantitative assessment of hepatic steatosis in liver transplants. Computers in Biology and Medicine 123, 103836 (2020)
Klauschen, F., Müller, K.-R., Binder, A., Bockmayr, M., Hägele, M., Seegerer, P., Wienert, S., Pruneri, G., Maria, S., Badve, S., Michiels, S., Nielsen, T.O., Adams, S., Savas, P., Symmans, F., Willis, S., Gruosso, T., Park, M., Haibe-Kains, B., Denkert, C.: Scoring of tumor-infiltrating lymphocytes: From visual estimation to machine learning. Seminars in Cancer Biology 52 (2018) https://doi.org/10.1016/j.semcancer.2018.07.001
Abousamra, S., Gupta, R., Hou, L., Batiste, R., Zhao, T., Shankar, A., Rao, A., Chen, C., Samaras, D., Kurc, T., et al: Deep learning-based mapping of tumor infiltrating lymphocytes in whole slide images of 23 types of cancer. Frontiers in oncology 11, 806603 (2022)
Campanella, G., Hanna, M.G., Geneslaw, L., Miraflor, A., Werneck Krauss Silva, V., Busam, K.J., Brogi, E., Reuter, V.E., Klimstra, D.S., Fuchs, T.J.: Clinical-grade computational pathology using weakly supervised deep learning on whole slide images. Nature medicine 25(8), 1301–1309 (2019)
Zhang, X., Zhu, X., Tang, K., Zhao, Y., Lu, Z., Feng, Q.: DDTNet: A dense dual-task network for tumor-infiltrating lymphocyte detection and segmentation in histopathological images of breast cancer. Medical Image Analysis 78, 102415 (2022)
Yosofvand, M., Khan, S.Y., Dhakal, R., Nejat, A., Moustaid-Moussa, N., Rahman, R.L., Moussa, H.: Automated Detection and Scoring of Tumor-Infiltrating Lymphocytes in Breast Cancer Histopathology Slides. Cancers 15(14), 3635 (2023)
Yu, X., Chen, P., Wu, D., Hassan, N., Li, G., Yan, J., Shi, H., Ye, Q., Han, Z.: Object Localization under Single Coarse Point Supervision (2022)
Jiménez, G., Racoceanu, D.: Deep learning for semantic segmentation vs. classification in computational pathology: application to mitosis analysis in breast cancer grading. Frontiers in bioengineering and biotechnology 7, 145 (2019)
Amgad, M., Salgado, R., Cooper, L.A.: A panoptic segmentation approach for tumor-infiltrating lymphocyte assessment: development of the MuTILs model and PanopTILs dataset. MedRxiv, 2022–01 (2022)
Zhang, X., Liu, K., Zhang, K., Li, X., Sun, Z., Wei, B.: SAMS-Net: Fusion of attention mechanism and multi-scale features network for tumor infiltrating lymphocytes segmentation. Math. Biosci. Eng 20, 2964–2979 (2023)
Hafiz, A.M., Bhat, G.M.: A survey on instance segmentation: state of the art. International journal of multimedia information retrieval 9(3), 171–189 (2020)
Nasir, E.S., Parvaiz, A., Fraz, M.M.: Nuclei and glands instance segmentation in histology images: a narrative review. Artificial Intelligence Review, 1–56 (2022)
Nam, S., Knag, M., Won, D., Chikontwe, P., Noh, B.-J., Go, H., Park, S.H.: Weakly-Supervised TILs Segmentation Based on Point Annotations Using Transfer Learning with Point Detector and Projected-Boundary Regressor. In: International Workshop on PRedictive Intelligence In MEdicine, pp. 115–125 (2022). Springer
Zafar, M.M., Rauf, Z., Sohail, A., Khan, A.R., Obaidullah, M., Khan, S.H., Lee, Y.S., Khan, A.: Detection of tumour infiltrating lymphocytes in CD3 and CD8 stained histopathological images using a two-phase deep CNN. Photodiagnosis and Photodynamic Therapy 37, 102676 (2022)
Miao, R., Toth, R., Zhou, Y., Madabhushi, A., Janowczyk, A.: Quick Annotator: an open-source digital pathology based rapid image annotation tool. The Journal of Pathology: Clinical Research 7(6), 542–547 (2021)
Turkki, R., Linder, N., Kovanen, P.E., Pellinen, T., Lundin, J.: Antibody-supervised deep learning for quantification of tumor-infiltrating immune cells in hematoxylin and eosin stained breast cancer samples. Journal of pathology informatics 7(1), 38 (2016)
Swiderska-Chadaj, Z., Pinckaers, H., Rijthoven, M., Balkenhol, M., Melnikova, M., Geessink, O., Manson, Q., Sherman, M., Polonia, A., Parry, J., et al: Learning to detect lymphocytes in immunohistochemistry with deep learning. Medical image analysis 58, 101547 (2019)
Tajbakhsh, N., Jeyaseelan, L., Li, Q., Chiang, J., Wu, Z., Ding, X.: Embracing imperfect datasets: A review of deep learning solutions for medical image segmentation. Medical Image Analysis 63, 101693 (2020) https://doi.org/10.1016/j.media.2020.101693
Meirelles, A., Kurc, T., Saltz, J., Teodoro, G.: Effective active learning in digital pathology: A case study in tumor infiltrating lymphocytes. Computer Methods and Programs in Biomedicine 220, 106828 (2022) https://doi.org/10.1016/j.cmpb.2022.106828
Korzynska, A., Roszkowiak, L., Zak, J., Siemion, K.: A review of current systems for annotation of cell and tissue images in digital pathology. Biocybernetics and Biomedical Engineering 41 (2021) https://doi.org/10.1016/j.bbe.2021.04.012
Amgad, M., Atteya, L.A., Hussein, H., Mohammed, K.H., Hafiz, E., Elsebaie, M.A., Alhusseiny, A.M., AlMoslemany, M.A., Elmatboly, A.M., Pappalardo, P.A., et al: NuCLS: A scalable crowdsourcing approach and dataset for nucleus classification and segmentation in breast cancer. GigaScience 11, 037 (2022)
Amgad, M., Elfandy, H., Hussein, H., Atteya, L.A., Elsebaie, M.A., Abo Elnasr, L.S., Sakr, R.A., Salem, H.S., Ismail, A.F., Saad, A.M., et al: Structured crowdsourcing enables convolutional segmentation of histology images. Bioinformatics 35(18), 3461–3467 (2019)
Montezuma, D., Oliveira, S.P., Neto, P.C., Oliveira, D., Monteiro, A., Cardoso, J.S., Macedo-Pinto, I.: Annotating for artificial intelligence applications in digital pathology: A practical guide for pathologists and researchers. Modern Pathology 36(4), 100086 (2023)
Priego Torres, B., Lobato-Delgado, B., Atienza-Cuevas, L., Morillo, D.: Deep learning-based instance segmentation for the precise automated quantification of digital breast cancer immunohistochemistry images. Expert Systems with Applications, 116471 (2022) https://doi.org/10.1016/j.eswa.2021.116471
Cardoso, M.J., Li, W., Brown, R., Ma, N., Kerfoot, E., Wang, Y., Murrey, B., Myronenko, A., Zhao, C., Yang, D., et al.: MONAI: An open-source framework for deep learning in healthcare. arXiv preprint arXiv:2211.02701 (2022)
Diaz-Pinto, A., Alle, S., Nath, V., Tang, Y., Ihsani, A., Asad, M., Pérez-García, F., Mehta, P., Li, W., Flores, M., et al.: MONAI Label: A framework for AI-assisted Interactive Labeling of 3D Medical Images. arXiv preprint arXiv:2203.12362 (2022)
Bankhead, P., Loughrey, M.B., Fernández, J.A., Dombrowski, Y., McArt, D.G., Dunne, P.D., McQuaid, S., Gray, R.T., Murray, L.J., Coleman, H.G., et al: QuPath: Open source software for digital pathology image analysis. Scientific reports 7(1), 1–7 (2017)
Hayakawa, T., Prasath, V.S., Kawanaka, H., Aronow, B.J., Tsuruoka, S.: Computational nuclei segmentation methods in digital pathology: a survey. Archives of Computational Methods in Engineering 28, 1–13 (2021)
Ding, R., Prasanna, P., Corredor, G., Barrera, C., Zens, P., Lu, C., Velu, P., Leo, P., Beig, N., Li, H., et al: Image analysis reveals molecularly distinct patterns of TILs in NSCLC associated with treatment outcome. NPJ precision oncology 6(1), 33 (2022)
Maibach, F., Sadozai, H., Seyed Jafari, S.M., Hunger, R.E., Schenk, M.: Tumor-infiltrating lymphocytes and their prognostic value in cutaneous melanoma. Frontiers in immunology 11, 2105 (2020)
Arvidsson, I., Overgaard, N.C., Marginean, F.-E., Krzyzanowska, A., Bjartell, A., Åström, K., Heyden, A.: Generalization of prostate cancer classification for multiple sites using deep learning. In: 2018 IEEE 15th International Symposium on Biomedical Imaging (ISBI 2018), pp. 191–194 (2018). IEEE
Janowczyk, A., Madabhushi, A.: Deep learning for digital pathology image analysis: A comprehensive tutorial with selected use cases. Journal of Pathology Informatics 7, 29 (2016) https://doi.org/10.4103/2153-3539.186902
Sirinukunwattana, K., Raza, S.E.A., Tsang, Y.-W., Snead, D.R., Cree, I.A., Rajpoot, N.M.: Locality sensitive deep learning for detection and classification of nuclei in routine colon cancer histology images. IEEE transactions on medical imaging 35(5), 1196–1206 (2016)
Naylor, P., Laé, M., Reyal, F., Walter, T.: Segmentation of nuclei in histopathology images by deep regression of the distance map. IEEE transactions on medical imaging 38(2), 448–459 (2018)
Saltz, J., Gupta, R., Hou, L., Kurc, T., Singh, P., Nguyen, V., Samaras, D., Shroyer, K.R., Zhao, T., Batiste, R., et al: Spatial organization and molecular correlation of tumor-infiltrating lymphocytes using deep learning on pathology images. Cell reports 23(1), 181–193 (2018)
Vu, Q.D., Graham, S., Kurc, T., To, M.N.N., Shaban, M., Qaiser, T., Koohbanani, N.A., Khurram, S.A., Kalpathy-Cramer, J., Zhao, T., et al.: Methods for Segmentation and Classification of Digital Microscopy Tissue Images. Frontiers in bioengineering and biotechnology, 53 (2019)
Graham, S., Vu, Q.D., Raza, S.E.A., Azam, A., Tsang, Y.W., Kwak, J.T., Rajpoot, N.: Hover-net: Simultaneous segmentation and classification of nuclei in multi-tissue histology images. Medical image analysis 58, 101563 (2019)
Verma, R., Kumar, N., Patil, A., Kurian, N.C., Rane, S., Graham, S., Vu, Q.D., Zwager, M., Raza, S.E.A., Rajpoot, N., et al: MoNuSAC2020: A multi-organ nuclei segmentation and classification challenge. IEEE Transactions on Medical Imaging 40(12), 3413–3423 (2021)
Gamper, J., Koohbanani, N., Benes, K., Graham, S., Jahanifar, M., Khurram, S., Azam, A., Hewitt, K., Rajpoot, N.: PanNuke Dataset Extension, Insights and Baselines. arXiv 2020. arXiv preprint arXiv:2003.10778 (2003)
Kumar, N., Verma, R., Sharma, S., Bhargava, S., Vahadane, A., Sethi, A.: A dataset and a technique for generalized nuclear segmentation for computational pathology. IEEE transactions on medical imaging 36(7), 1550–1560 (2017)
Graham, S., Jahanifar, M., Azam, A., Nimir, M., Tsang, Y.-W., Dodd, K., Hero, E., Sahota, H., Tank, A., Benes, K., et al: Lizard: a large-scale dataset for colonic nuclear instance segmentation and classification. In: Proceedings of the IEEE/CVF International Conference on Computer Vision, pp. 684–693 (2021)
Sirinukunwattana, K., Pluim, J.P., Chen, H., Qi, X., Heng, P.-A., Guo, Y.B., Wang, L.Y., Matuszewski, B.J., Bruni, E., Sanchez, U., et al: Gland segmentation in colon histology images: The glas challenge contest. Medical image analysis 35, 489–502 (2017)
Graham, S., Chen, H., Gamper, J., Dou, Q., Heng, P.-A., Snead, D., Tsang, Y.W., Rajpoot, N.: MILD-Net: Minimal information loss dilated network for gland instance segmentation in colon histology images. Medical image analysis 52, 199–211 (2019)
Da, Q., Huang, X., Li, Z., Zuo, Y., Zhang, C., Liu, J., Chen, W., Li, J., Xu, D., Hu, Z., Yi, H., Guo, Y., Wang, Z., Chen, L., Zhang, L., He, X., Zhang, X., Mei, K., Zhu, C., Lu, W., Shen, L., Shi, J., Li, J., S, S., Krishnamurthi, G., Yang, J., Lin, T., Song, Q., Liu, X., Graham, S., Bashir, R.M.S., Yang, C., Qin, S., Tian, X., Yin, B., Zhao, J., Metaxas, D.N., Li, H., Wang, C., Zhang, S.: Digestpath: A benchmark dataset with challenge review for the pathological detection and segmentation of digestive-system. Medical Image Analysis 80, 102485 (2022) https://doi.org/10.1016/j.media.2022.102485
Akbar, S., Peikari, M., Salama, S., Panah, A.Y., Nofech-Mozes, S., Martel, A.L.: Automated and manual quantification of tumour cellularity in digital slides for tumour burden assessment. Scientific reports 9(1), 14099 (2019)
Saltz, J., Gupta, R., Hou, L., Kurc, T., Singh, P., Nguyen, V., Samaras, D., Shroyer, K., Zhao, T., Batiste, R., Arnam, J., Shmulevich, I., Rao, A., Lazar, A., Sharma, A., Thorsson, V.: Spatial organization and molecular correlation of tumor-infiltrating lymphocytes using deep learning on pathology images. Cell reports 23, 181–1937 (2018) https://doi.org/10.1016/j.celrep.2018.03.086
Graham, S., Jahanifar, M., Vu, Q.D., Hadjigeorghiou, G., Leech, T., Snead, D., Raza, S.E.A., Minhas, F., Rajpoot, N.: CoNIC: Colon Nuclei Identification and Counting Challenge 2022. arXiv preprint arXiv:2111.14485 (2021)
Graham, S., Vu, Q.D., Jahanifar, M., Weigert, M., Schmidt, U., Zhang, W., Zhang, J., Yang, S., Xiang, J., Wang, X., et al.: CoNIC Challenge: Pushing the Frontiers of Nuclear Detection, Segmentation, Classification and Counting. arXiv preprint arXiv:2303.06274 (2023)
Shephard, A., Jahanifar, M., Wang, R., Dawood, M., Graham, S., Sidlauskas, K., Khurram, S.A., Rajpoot, N., Raza, S.E.A.: TIAger: Tumor-Infiltrating Lymphocyte Scoring in Breast Cancer for the TiGER Challenge. arXiv preprint arXiv:2206.11943 (2022)
Hosseini, M.S., Chan, L., Tse, G., Tang, M., Deng, J., Norouzi, S., Rowsell, C., Plataniotis, K.N., Damaskinos, S.: Atlas of digital pathology: A generalized hierarchical histological tissue type-annotated database for deep learning. In: Proceedings of the IEEE/CVF Conference on Computer Vision and Pattern Recognition, pp. 11747–11756 (2019)
Shvetsov, N., Grønnesby, M., Pedersen, E., Møllersen, K., Busund, L.-T.R., Schwienbacher, R., Bongo, L.A., Kilvaer, T.K.: A pragmatic machine learning approach to quantify tumor-infiltrating lymphocytes in whole slide images. Cancers 14(12), 2974 (2022)
Rakaee, M., Kilvaer, T.K., Dalen, S.M., Richardsen, E., Paulsen, E.-E., Hald, S.M., Al-Saad, S., Andersen, S., Donnem, T., Bremnes, R.M., et al: Evaluation of tumor-infiltrating lymphocytes using routine H&E slides predicts patient survival in resected non–small cell lung cancer. Human pathology 79, 188–198 (2018)
Fassler, D.J., Torre-Healy, L.A., Gupta, R., Hamilton, A.M., Kobayashi, S., Van Alsten, S.C., Zhang, Y., Kurc, T., Moffitt, R.A., Troester, M.A., et al: Spatial characterization of tumor-infiltrating lymphocytes and breast cancer progression. Cancers 14(9), 2148 (2022)
Tomczak, K., Czerwińska, P., Wiznerowicz, M.: Review The Cancer Genome Atlas (TCGA): an immeasurable source of knowledge. Contemporary Oncology/Współczesna Onkologia 2015(1), 68–77 (2015)
Blei, D.M., Ng, A.Y., Jordan, M.I.: Latent dirichlet allocation. Journal of machine Learning research 3(Jan), 993–1022 (2003)
Yijun, G., Tian, X.: Study on keyword extraction with LDA and TextRank combination. Data Analysis and Knowledge Discovery 30(7), 41–47 (2014)
Song, Y., Pan, S., Liu, S., Zhou, M.X., Qian, W.: Topic and keyword re-ranking for LDA-based topic modeling. Proceedings of the 18th ACM conference on Information and knowledge management, 1757–1760 (2009)
Inoue, T., Yagi, Y.: Color standardization and optimization in whole slide imaging. Clinical and Diagnostic Pathology 4 (2020) https://doi.org/10.15761/CDP.1000139
Badano, A., Revie, C., Casertano, A., Cheng, W.-C., Green, P., Kimpe, T., Krupinski, E., Sisson, C., Skrøvseth, S., Treanor, D., et al: Consistency and standardization of color in medical imaging: a consensus report. Journal of digital imaging 28, 41–52 (2015)
Yagi, Y.: Color standardization and optimization in Whole Slide Imaging. Diagnostic pathology 6 Suppl 1, 15 (2011) https://doi.org/10.1186/1746-1596-6-S1-S15
Mani, N., Schalper, K., Hatzis, C., Saglam, O., Tavassoli, F., Butler, M., Chagpar, A., Pusztai, L., Rimm, D.: Quantitative assessment of the spatial heterogeneity of tumor-infiltrating lymphocytes in breast cancer:. Breast Cancer Research 18 (2016) https://doi.org/10.1186/s13058-016-0737-x
Gupta, R., Hou, L., Abousamra, S., Fassler, D., Torre-Healy, L., Moffitt, R., Kurc, T., Samaras, D., Batiste, R., Zhao, T., Rao, A., Dyke, A., Sharma, A., Bremer, E., Almeida, J., Saltz, J.: Utilizing automated breast cancer detection to identify spatial distributions of tumor infiltrating lymphocytes in invasive breast cancer. The American Journal of Pathology 190 (2020) https://doi.org/10.1016/j.ajpath.2020.03.012
Camp, R., Feezor, R., Kasraeian, A., Cendan, J., Schell, S., Wilkinson, E., Copeland, E., Lind, D.: Sentinel lymph node biopsy for ductal carcinoma in situ: an evolving approach at the University of Florida. The breast journal 11, 394–7 (2005) https://doi.org/10.1111/j.1075-122X.2005.00128.x
Olapade-Olaopa, E., MacKay, E., Habib, F.: Variability of immunohistochemical reactivity on stored paraffin slides. Journal of clinical pathology 51, 943 (1999) https://doi.org/10.1136/jcp.51.12.943b
Libard, S., Cerjan, D., Alafuzoff, I.: Characteristics of the tissue section that influence the staining outcome in immunohistochemistry. Histochemistry and Cell Biology 151, 91–96 (2019)
Kim, S.-W., Roh, J., Park, C.-S.: Immunohistochemistry for pathologists: protocols, pitfalls, and tips. Journal of pathology and translational medicine 50(6), 411–418 (2016)
Tan, W., Nerurkar, S., Cai, H., Ng, H.H.M., Wu, D., Wee, Y., Lim, J.C.T., Yeong, J., Lim, T.: Overview of multiplex immunohistochemistry/immunofluorescence techniques in the era of cancer immunotherapy. Cancer Communications 40 (2020) https://doi.org/10.1002/cac2.12023
Conde-Sousa, E., Vale, J., Feng, M., Xu, K., Wang, Y., Della Mea, V., La Barbera, D., Montahaei, E., Soleymani, M., Turzynski, A., Gildenblat, J., Klaiman, E., Hong, Y., Aresta, G., Araújo, T., Aguiar, P., Eloy, C., Polónia, A.: HEROHE Challenge: Predicting HER2 Status in Breast Cancer from Hematoxylin-Eosin Whole-Slide Imaging. Journal of Imaging 8, 213 (2022) https://doi.org/10.3390/jimaging8080213
Funding
Open Access funding provided thanks to the CRUE-CSIC agreement with Springer Nature. This work was supported by BosomShield, a project that has received funding from Marie Skłodowska-Curie Doctoral Networks Actions (HORIZON-MSCA-2021-DN-01-01) under grant agreement 101073222. Further support was provided by SCARLET, a project funded by Proyectos Estratégicos Orientados a la Transición Ecológica y a la Transición Digital, from the 2021 call of the Ministerio de Ciencia e Innovación, with grant number TED2021-130081B-C22 and funding from NextGenerationEU. This work was also partially supported by the Department Strategic Plan of the University of Udine-Interdepartmental Project on Artificial Intelligence (2021-25).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Fiorin, A., López Pablo, C., Lejeune, M. et al. Enhancing AI Research for Breast Cancer: A Comprehensive Review of Tumor-Infiltrating Lymphocyte Datasets. J Digit Imaging. Inform. med. (2024). https://doi.org/10.1007/s10278-024-01043-8
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10278-024-01043-8