Abstract
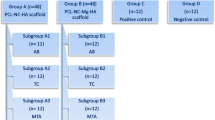
Scaffolds are crucial elements for dental pulp regeneration. Most of the currently used scaffolds in regenerative endodontic procedures (REPs) are unsuitable for chairside clinical use. This study aimed to evaluate the effect of an injectable synthetic scaffold (Restylane Lyft) on human bone marrow mesenchymal stem cell (hBMSC) viability, proliferation, and osteo/dentinogenic differentiation in a regenerative endodontic organotype model (REM). hBMSC were loaded in an REM either alone (hBMSC group) or mixed with the Restylane Lyft scaffold (Restylane/hBMSC group) and cultured in basal culture medium (n = 9/group). hMSC on culture plates served as controls. Cell viability and proliferation were measured using AlamarBlue assay. The loaded REM was cultured in an osteogenic differentiation medium to measure alkaline phosphatase activity (ALP) and examine the expression of the osteo/dentinogenic markers using real-time reverse transcriptase polymerase chain reaction. Cell viability in all groups increased significantly over 5 days. The Restylane/hBMSC group showed significantly higher ALP activity and dentin sialophosphoprotein, osteocalcin, and bone sialoprotein genes expression than the hBMSC and the control groups. Restylane Lyft, a hyaluronic acid (HA) injectable, FDA-approved hydrogel, maintained cell viability and proliferation and promoted osteo/dentinogenic differentiation of hBMSC when cultured in an REM. Henceforth, it could be a promising chairside scaffold material for REPs.




Similar content being viewed by others
References
Banchs F, Trope M. Revascularization of immature permanent teeth with apical periodontitis: new treatment protocol? J Endod. 2004;30(4):196–200. https://doi.org/10.1097/00004770-200404000-00003.
Murray PE, Garcia-Godoy F, Hargreaves KM. Regenerative endodontics: a review of current status and a call for action. J Endod. 2007;33(4):377–90. https://doi.org/10.1016/j.joen.2006.09.013.
Murray PE. Platelet-rich plasma and platelet-rich fibrin can induce apical closure more frequently than blood-clot revascularization for the regeneration of immature permanent teeth: a meta-analysis of clinical efficacy. Front Bioeng Biotechnol. 2018;6:139. https://doi.org/10.3389/fbioe.2018.00139.
Lenzi R, Trope M. Revitalization procedures in two traumatized incisors with different biological outcomes. J Endod. 2012;38(3):411–4. https://doi.org/10.1016/j.joen.2011.12.003.
Kharkar PM, Kiick KL, Kloxin AM. Designing degradable hydrogels for orthogonal control of cell microenvironments. Chem Soc Rev. 2013;42(17):7335–72. https://doi.org/10.1039/c3cs60040h.
Abbass MMS, El-Rashidy AA, Sadek KM, Moshy SE, Radwan IA, Rady D et al. Hydrogels and Dentin-Pulp Complex Regeneration: From the Benchtop to Clinical Translation. Polymers (Basel). 2020;12(12). doi:https://doi.org/10.3390/polym12122935.
FDA. Medical Device Definition. In: http://www.fda.gov/MedicalDevices/DeviceRegulationandGuidance/Overview/ClassifyYourDevice/ucm051512.htm, Food and Drug Administration. Last Accessed: February 23, 2022.
Inuyama Y, Kitamura C, Nishihara T, Morotomi T, Nagayoshi M, Tabata Y, et al. Effects of hyaluronic acid sponge as a scaffold on odontoblastic cell line and amputated dental pulp. J Biomed Mater Res B Appl Biomater. 2010;92(1):120–8. https://doi.org/10.1002/jbm.b.31497.
Coimbra P, Alves P, Valente TAM, Santos R, Correia IJ, Ferreira P. Sodium hyaluronate/chitosan polyelectrolyte complex scaffolds for dental pulp regeneration: Synthesis and characterization. Int J Biol Macromol. 2011;49(4):573–9. https://doi.org/10.1016/j.ijbiomac.2011.06.011.
Chen KL, Yeh YY, Lung J, Yang YC, Yuan K. Mineralization effect of hyaluronan on dental pulp cells via CD44. J Endod. 2016;42(5):711–6. https://doi.org/10.1016/j.joen.2016.01.010.
Silva CR, Babo PS, Gulino M, Costa L, Oliveira JM, Silva-Correia J, et al. Injectable and tunable hyaluronic acid hydrogels releasing chemotactic and angiogenic growth factors for endodontic regeneration. Acta Biomater. 2018;77:155–71. https://doi.org/10.1016/j.actbio.2018.07.035.
Bronckers AL, Lyaruu DM, Woltgens JH. Immunohistochemistry of extracellular matrix proteins during various stages of dentinogenesis. Connect Tissue Res. 1989;22(1–4):65–70.
Cassidy N, Fahey M, Prime SS, Smith AJ. Comparative analysis of transforming growth factor-beta isoforms 1–3 in human and rabbit dentine matrices. Arch Oral Biol. 1997;42(3):219–23. https://doi.org/10.1016/s0003-9969(96)00115-x.
Chrepa V, Austah O, Diogenes A. Evaluation of a commercially available hyaluronic acid hydrogel (restylane) as injectable scaffold for dental pulp regeneration: an in vitro evaluation. J Endod. 2017;43(2):257–62. https://doi.org/10.1016/j.joen.2016.10.026.
Sundaram H, Voigts BOB, Beer K, Meland M. Comparison of the rheological properties of viscosity and elasticity in two categories of soft tissue fillers: calcium hydroxylapatite and hyaluronic acid. Dermatol Surg. 2010;36(Suppl 3):1859–65. https://doi.org/10.1111/j.1524-4725.2010.01743.x.
Trevino EG, Patwardhan AN, Henry MA, Perry G, Dybdal-Hargreaves N, Hargreaves KM, et al. Effect of irrigants on the survival of human stem cells of the apical papilla in a platelet-rich plasma scaffold in human root tips. J Endod. 2011;37(8):1109–15. https://doi.org/10.1016/j.joen.2011.05.013.
AAE. Considerations for a regenerative procedure. Available from: http://www.aae.org/uploadedfiles/publications_and_research/research/currentregenerativeendodonticconsiderations.pdf (accessed on: 23, February 2022).
White JM, Goodis HE, Marshall SJ, Marshall GW. Sterilization of teeth by gamma radiation. J Dent Res. 1994;73(9):1560–7. https://doi.org/10.1177/00220345940730091201.
Sperandio M, Souza JB, Oliveira DT. Effect of gamma radiation on dentin bond strength and morphology. Braz Dent J. 2001;12(3):205–8.
Western JS, Dicksit DD. A systematic review of randomized controlled trials on sterilization methods of extracted human teeth. J Conserv Dent. 2016;19(4):343–6. https://doi.org/10.4103/0972-0707.186457.
Simonsen JL, Rosada C, Serakinci N, Justesen J, Stenderup K, Rattan SI, et al. Telomerase expression extends the proliferative life-span and maintains the osteogenic potential of human bone marrow stromal cells. Nat Biotechnol. 2002;20(6):592–6. https://doi.org/10.1038/nbt0602-592.
Galler KM, Hartgerink JD, Cavender AC, Schmalz G, D’Souza RN. A customized self-assembling peptide hydrogel for dental pulp tissue engineering. Tissue Eng Part A. 2012;18(1–2):176–84. https://doi.org/10.1089/ten.tea.2011.0222.
Galler KM, D’Souza RN, Hartgerink JD, Schmalz G. Scaffolds for dental pulp tissue engineering. Adv Dent Res. 2011;23(3):333–9. https://doi.org/10.1177/0022034511405326.
Huang GT, Gronthos S, Shi S. Mesenchymal stem cells derived from dental tissues vs. those from other sources: their biology and role in regenerative medicine. J Dent Res. 2009;88(9):792–806. doi:https://doi.org/10.1177/0022034509340867.
Murakami M, Hayashi Y, Iohara K, Osako Y, Hirose Y, Nakashima M. Trophic effects and regenerative potential of mobilized mesenchymal stem cells from bone marrow and adipose tissue as alternative cell sources for pulp/dentin regeneration. Cell Transplant. 2015;24(9):1753–65. https://doi.org/10.3727/096368914x683502.
Shi S, Robey PG, Gronthos S. Comparison of human dental pulp and bone marrow stromal stem cells by cDNA microarray analysis. Bone. 2001;29(6):532–9. https://doi.org/10.1016/s8756-3282(01)00612-3.
Abdallah BM, Haack-Sørensen M, Burns JS, Elsnab B, Jakob F, Hokland P, et al. Maintenance of differentiation potential of human bone marrow mesenchymal stem cells immortalized by human telomerase reverse transcriptase gene despite [corrected] extensive proliferation. Biochem Biophys Res Commun. 2005;326(3):527–38. https://doi.org/10.1016/j.bbrc.2004.11.059.
Al-Nbaheen M, Vishnubalaji R, Ali D, Bouslimi A, Al-Jassir F, Megges M, et al. Human stromal (mesenchymal) stem cells from bone marrow, adipose tissue and skin exhibit differences in molecular phenotype and differentiation potential. Stem Cell Rev Rep. 2013;9(1):32–43. https://doi.org/10.1007/s12015-012-9365-8.
Twine NA, Harkness L, Adjaye J, Aldahmash A, Wilkins MR, Kassem M. Molecular phenotyping of telomerized human bone marrow skeletal stem cells reveals a genetic program of enhanced proliferation and maintenance of differentiation responses. JBMR Plus. 2018;2(5):257–67. https://doi.org/10.1002/jbm4.10050.
Gronthos S, Robey P, Boyde A, Shi S, editors. Human dental pulp stem cells (DPSCs): Characterization and developmental potential. J Bone Mineral Res; 2001: Wiley Malden.
Otaki S, Ueshima S, Shiraishi K, Sugiyama K, Hamada S, Yorimoto M, et al. Mesenchymal progenitor cells in adult human dental pulp and their ability to form bone when transplanted into immunocompromised mice. Cell Biol Int. 2007;31(10):1191–7. https://doi.org/10.1016/j.cellbi.2007.04.001.
Miura M, Gronthos S, Zhao M, Lu B, Fisher LW, Robey PG, et al. SHED: stem cells from human exfoliated deciduous teeth. Proc Natl Acad Sci USA. 2003;100(10):5807–12. https://doi.org/10.1073/pnas.0937635100.
Streichan SJ, Hoerner CR, Schneidt T, Holzer D, Hufnagel L. Spatial constraints control cell proliferation in tissues. Proc Natl Acad Sci USA. 2014;111(15):5586–91. https://doi.org/10.1073/pnas.1323016111.
Edmondson R, Broglie JJ, Adcock AF, Yang L. Three-dimensional cell culture systems and their applications in drug discovery and cell-based biosensors. Assay Drug Dev Technol. 2014;12(4):207–18. https://doi.org/10.1089/adt.2014.573.
Cardoso DA, Ulset AS, Bender J, Jansen JA, Christensen BE, Leeuwenburgh SC. Effects of physical and chemical treatments on the molecular weight and degradation of alginate-hydroxyapatite composites. Macromol Biosci. 2014;14(6):872–80. https://doi.org/10.1002/mabi.201300415.
Erisken C, Kalyon DM, Zhou J, Kim SG, Mao JJ. Viscoelastic properties of dental pulp tissue and ramifications on biomaterial development for pulp regeneration. J Endod. 2015;41(10):1711–7. https://doi.org/10.1016/j.joen.2015.07.005.
Nath SD, Abueva C, Kim B, Lee BT. Chitosan-hyaluronic acid polyelectrolyte complex scaffold crosslinked with genipin for immobilization and controlled release of BMP-2. Carbohydr Polym. 2015;115:160–9. https://doi.org/10.1016/j.carbpol.2014.08.077.
Cameron AR, Frith JE, Gomez GA, Yap AS, Cooper-White JJ. The effect of time-dependent deformation of viscoelastic hydrogels on myogenic induction and Rac1 activity in mesenchymal stem cells. Biomaterials. 2014;35(6):1857–68. https://doi.org/10.1016/j.biomaterials.2013.11.023.
Mintz BR, Cooper JA Jr. Hybrid hyaluronic acid hydrogel/poly(ɛ-caprolactone) scaffold provides mechanically favorable platform for cartilage tissue engineering studies. J Biomed Mater Res A. 2014;102(9):2918–26. https://doi.org/10.1002/jbm.a.34957.
Wang J, Ma H, Jin X, Hu J, Liu X, Ni L, et al. The effect of scaffold architecture on odontogenic differentiation of human dental pulp stem cells. Biomaterials. 2011;32(31):7822–30. https://doi.org/10.1016/j.biomaterials.2011.04.034.
Smith AJ, Tobias RS, Cassidy N, Begue-Kirn C, Ruch JV, Lesot H. Influence of substrate nature and immobilization of implanted dentin matrix components during induction of reparative dentinogenesis. Connect Tissue Res. 1995;32(1–4):291–6.
Acknowledgements
The authors would like to thank Mr.Nasr AlMuflahi, Community Dentistry Department, College of Dentistry, King Saud University, for conducting the statistical analysis of the data presented.
Funding
This research received support from Researchers Supporting Project number (RSP2022R471), King Saud University, Riyadh, Saudi Arabia.
Author information
Authors and Affiliations
Contributions
Conceptualization, NA, and DA; methodology, NA, and NA; validation, Norah A. AlHowaish, and Nihal A. AlMuraikhi; formal analysis, Nihal A. AlMuraikhi; investigation, Norah A. AlHowaish; writing—original draft preparation, Norah A. AlHowaish; writing—review and editing, Dina I. AlSudani; supervision, Dina I. AlSudani, and Nihal A. AlMuraikhi; All authors have read and agreed to the submitted version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors deny any conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
AlHowaish, N.A., AlSudani, D.I. & AlMuraikhi, N.A. Evaluation of a hyaluronic acid hydrogel (Restylane Lyft) as a scaffold for dental pulp regeneration in a regenerative endodontic organotype model. Odontology 110, 726–734 (2022). https://doi.org/10.1007/s10266-022-00710-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10266-022-00710-y