Abstract
This research provides insight into a unique salt tolerance mechanism of Vigna riukiuensis. V. riukiuensis is one of the salt-tolerant species identified from the genus Vigna. We have previously reported that V. riukiuensis accumulates a higher amount of sodium in the leaves, whereas V. nakashimae, a close relative of V. riukiuensis, suppresses sodium allocation to the leaves. We first suspected that V. riukiuensis would have developed vacuoles for sodium sequestration, but there were no differences compared to a salt-sensitive species V. angularis. However, many starch granules were observed in the chloroplasts of V. riukiuensis. In addition, forced degradation of leaf starch by shading treatment resulted in no radio-Na (22Na) accumulation in the leaves. We performed SEM–EDX to locate Na in leaf sections and detected Na in chloroplasts of V. riukiuensis, especially around the starch granules but not in the middle of. Our results could provide the second evidence of the Na-trapping system by starch granules, following the case of common reed that accumulates starch granule at the shoot base for binding Na.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Salt tolerance has been one of the most important issues in plant science as soil salinity is a major constraint of crop production. In addition, given a rapid depletion of ground water plus the rapid growth of global population, there is a concern that demands for drinking water may compete with those for food production (Wada et al. 2010). Thus, there is a growing demand for salt-tolerant crops, which can be grown with salinized water (Panta et al. 2014).
To this goal, we have screened genetic resources of wild Vigna species and identified several species that are highly tolerant to salt stress (Iseki et al. 2016; Yoshida et al. 2016). The genus Vigna is a reservoir of diversity and many species are adapted to harsh environments, such as marine beach, desert, limestone karsts and marshes (Tomooka et al. 2014; van Zonneveld et al. 2020). As expected, the species collected from coastal areas presented high tolerance to salt (Iseki et al. 2016).
The following studies on sodium allocation have revealed that Vigna riukiuensis (Ohwi) Ohwi & H.Ohashi accumulates a relatively higher amount of sodium in the leaves, whereas Vigna nakashimae (Ohwi) Ohwi & H.Ohashi, a close relative of V. riukiuensis, suppresses sodium accumulation to the leaves (Noda et al. 2022; Yoshida et al. 2016). Thus, we have hypothesized that V. riukiuensis has a mechanism of salt-includer, which isolates sodium into vacuoles to lower sodium concentration in cytoplasm.
To confirm this hypothesis, in this study, we first observed vacuoles of the leaf cells to see whether there are any structural differences between V. riukiuensis and other species. However, we could not find any characteristic features in the vacuoles. Instead, we found an abundance of starch granules in the chloroplast of V. riukiuensis leaves. Since there has been a report that common reed accumulates starch granules that bind Na at the shoot base (Kanai et al. 2007), we were intrigued to test whether the starch granules in V. riukiuensis also have ability to bind Na. To do so, we performed further experiments including element mapping.
Materials and methods
Plant material and growth conditions
All plant seeds were provided by the NARO Genebank in Tsukuba, Japan (https://www.gene.affrc.go.jp/index_en.php). Seeds were germinated on Seramis clay (Westland Deutschland GmbH, Mogendorf, Germany) for 1 week and then transferred to hydroponic solution in a growth chamber (Light: 28 °C for 14 h and Dark: 25 °C for 10 h. Light intensity 500 µM−1 s−1 m−2) until autoradiographic imaging. The hydroponic solution contained a diluted nutrient solution of a 1:1 ratio of OAT House No.1 (1.5 g L−1): OAT House No.2 (1 g L−1) (Otsuka Chemical Co, Japan), which contained 18.6 mEq L−1 N, 5.1 mEq L−1 P, 8.6 mEq L−1 K, 8.2 mEq L−1 Ca and 3.0 mEq L−1 Mg.
Visualization of 22Na
Pre-cultured plants were transplanted to a new hydroponic solution containing 5 kBq 22Na (PerkinElmer, USA) with non-radioactive 100 mM 23NaCl. After adding the radio-isotope, plants were incubated again in a long-day condition (Light: 28 °C for 14 h and Dark: 25 °C for 10 h. Light intensity 200 µmol s−1 m−2) for 3 days. After incubation, we carefully washed the roots and then enclosed the whole plant body into a plastic bag, and exposed it to a Storage Phosper Screen (BAS-IP-MS-2025E, GE Healthcare, UK) in Amersham exposure cassettes (GE Healthcare, UK) for 24 h. We then scanned the exposed screen with a laser imaging scanner Typhoon FLA-9500 (GE Healthcare, UK). To arrange radioactive intensity equally at each image, photo-stimulated luminescence and contrast were equalized by Multi Gauge version 3.0 (Fujifilm, Japan). All the experiments were independently done on more than three biological replicates.
ICP-MS
We germinated the seeds on Seramis clay, cultivated for 1 week and then transferred 4 plants of each species to hydroponic solution (as described above) in a growth chamber (Light: 28 °C for 14 h and Dark: 24 °C for10 h). When the 3rd leaves had fully expanded, we transferred the plants to hydroponic culture with 100 mM NaCl for 2 days. After incubation, we separately collected the 1st and 2nd leaves and dried at 50 °C for 3 days. The leaves were digested with 200 µL 69% HNO3 at 90 °C for 0.5 h. The digestate was diluted to 1-in-140 with Milli-Q water and inductively coupled plasma-mass spectrometry (ICP-MS, NexION 350S, PerkinElmer, Waltham, MA, USA) determined the contents of Na. The Tukey HSD test was used to compare differences in the measured variables of leaf Na and K concentration, respectively. Differences were significant when p < 0.05.
Starch staining and correlation between starch and Na accumulation with shading in V. riukiuensis
Iodine staining
At the beginning of the treatment, we grew Vigna angularis (Willd.) Ohwi & H.Ohashi,, V. nakashimae and V. riukiuensis in hydroponic solution for 7 days, 14 days and 21 days, respectively, to unify the growth stage of the plants. Plants were grown in 100 mM NaCl hydroponic solution or non-NaCl solution for 3 days. After incubation, we harvested fresh leaves, immediately immersed them in hot water for 10 min and decolorized in 99.5% ethanol for 5 min. Finally, samples were immersed 1/50 iodine solution (20 g L−1 KI, 10 g Iodine) for 10 min.
Shading experiment
V. riukiuensis plants were grown in hydroponic solution for 21 days. The middle leaflets of the 1st, 2nd and 3rd leaves were masked with aluminum foil (shaded) or cling film (non-shaded control) at 2 pm. Plants were transferred to 100 mM 22NaCl solution (5 kBq) for 72 h under long-day condition. After 22Na treatment, 22Na localization was visualized using the method described above. For testing the effect of salt stress on starch degradation, we transferred the 3-week-old plants to 100 mM 23NaCl solution for 24 h and then covered the leaflets with aluminum foil or cling film at 2 pm and kept the plants in 100 mM NaCl for another 24 h. Leaves were then collected and processed for iodine–starch staining as described above.
Electron microscopy and SEM–EDX
We treated V. angularis, V. nakashimae and V. riukiuensis with 100 mM NaCl for 3 days. We cut the leaves into 5 × 5 mm2 pieces, and the samples were fixed with 4% (w/v) paraformaldehyde and 2% (v/v) glutaraldehyde in 50 mM cacodylate buffer for 2.5 h at room temperature. The samples were washed six times by the same buffer and postfixed with 1% osmium tetroxide in 50 mM cacodylate buffer for 2 h. After washing with double distilled water, the samples were dehydrated in a methanol series (25, 50, 75, 90, 100%) and substituted to methanol:propylene oxide (1:1) to 100% propylene oxide. Next, the samples were substituted in propylene oxide: Epon812 series (3:1, 1:1, 1:3) and finally embedded in 100% Epon812 resin (TAAB, UK). Semi-thin sections (1 or 2 µm) were cut with a diamond knife on an ultramicrotome (EM UC7, Leica Microsystems, Germany). To test whether there are any structural differences between each species, 1 µm thick sections were observed by a field-emission scanning electron microscope (FE-SEM). Sections were dried on glass slides and stained with 0.4% uranyl acetate and lead citrate solution. Sections were coated with osmium tetroxide with an osmium coater (HPC-1SW, Vacuum device, Japan), then observed by FE-SEM (SU8220, Hitachi High-Tech, Japan) at accelerating voltage 5 kV with an yttrium aluminum garnet backscattered electron detector. For scanning electron microscope-energy dispersive x-ray spectrometry (SEM–EDX), 2 µm thickness sections were dried on carbon tape at 60 °C and set on the scanning electron microscope (SEM) stub. The Na imaging condition was high vacuum and acceleration voltage mode as follows: Acceleration voltage, 15 kV; Spot intensity, 80; Beam exposure time, 1 h. As beam irradiation causes a slight elongation of the sections, electron microscopy images were acquired after beam irradiation in low vacuum and acceleration voltages. Spectral acquisition of Na and electron microscope images were acquired by EMAX Evolution (version EMAX 2.2 SP2, HORIBA, Japan) and SU3500 (Hitachi High-Tech, Japan), respectively. Spectral data was converted into Na mapping image by ImageJ version 1.51j8 (National Institutes of Health, USA). We checked the intensity of the Na signal in energy dispersive X-ray spectroscopy (EDX) images by quantitative analysis provided in the EMAX Evolution. Sodium mapping and signal intensity in these species were tested by more than three biological replicates.
Acclimation test
To test the effect of acclimation on Na allocation, plants were grown for 2 weeks in hydroponic culture without NaCl, transferred to a culture with 50 mM NaCl for 3 days, and then transferred to that with 100 mM NaCl (with 5 kBq 22Na) for another 3 days. After the treatment, plants were collected and processed for autoradiography as described above.
Acute salt stress
To test the effect of acute salt stress, plants grown in hydroponic culture without NaCl were transferred to that with 200 mM NaCl for 3 days. Plants were then visually evaluated for salt injury.
Results
Sodium allocation in V. riukiuensis and its relatives
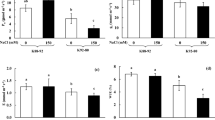
To confirm that V. riukiuensis accumulates higher amount of Na in the leaves, we evaluated Na allocation in V. angularis, V. nakashimae and V. riukiuensis by autoradiograph and mass spectrometry. The results reproduced our previous observations (Noda et al. 2022; Yoshida et al. 2016), where Na allocation to the leaves were low in V. nakashimae but high in V. riukiuensis (Fig. 1). V. angularis, which is sensitive to salt stress, allocated more Na in the lower leaves than in the higher leaves.
Na allocation in the plants. a–c Bright field photograph (left) and autoradiography (right) of the plants fed with 22Na. The luminance of 22Na distribution was standardized for each accession. Color change from blue to red indicates 22Na accumulation. White bars indicate 1 cm. d Na concentrations in each leaf. Circles, X and error bars indicate values of each replicate, means and standard deviation, respectively. L1, L2 and L3 indicate the 1st, 2nd and 3rd leaves, respectively. ang, nak and riu indicate V. angularis, V. nakashimae and V. riukiuensis, respectively. Means not sharing the same alphabet are significantly different (Tukey HSD p < 0.05)
Electron microscope image of leaf cells
Because V. riukiuensis accumulated a higher amount of Na in the leaves, we considered that it isolated Na to the vacuoles. To test whether any specific features are observable in the vacuoles of V. riukiuensis, we obtained electron microscope images on the leaf cells of V. angularis and V. riukiuensis.
However, we could not find any specific features in vacuoles of V. riukiuensis. Regardless of salt stress, the vacuoles were fully developed and occupied most of the cell compartments in both the species (Fig. 2). The only difference we found between the two species was that V. riukiuensis contained well-developed starch granules in the chloroplasts whereas V. angularis did not (Fig. 2).
Iodine–starch staining
To confirm that V. riukiuensis accumulates starch granules in the leaf cells, we conducted iodine–starch staining on the leaves of V. angularis, V. nakashimae and V. riukiuensis (Fig. 3). As a result, we observed strong staining in the leaves of V. riukiuensis but not in those of V. angularis, confirming high starch content in V. riukiuensis but low in V. angularis. However, we also observed strong staining in the leaves of V. nakashimae. Thus, it did not seem that the Na allocation to the leaves in V. riukiuensis was because of the higher content of starch. The starch granule experiment was conducted at the same time (2:00 p.m.), taking into account the difference in accumulation due to the day-night cycle.
Suppressed Na allocation to shaded leaves of V. riukiuensis
However, we still suspected that starch granules in V. riukiuensis positively affected Na allocation to the leaves. To test the hypothesis, we shaded certain leaflets of V. riukiuensis plants for 24 h to have all the starch granules degraded (Fig. 4). We then fed the plants with 22Na and took an autoradiograph to visualize Na allocation. As a control experiment, we wrapped the leaflets of the same positions with cling film to minimize the effect of covering, which might reduce Na accumulation by decreasing transpiration.
The results were remarkable. The autoradiography after 22Na treatment clearly showed that the shaded leaflets did not accumulate 22Na, while all other leaves (including “wrapped” leaflets in control) exhibited strong indication of 22Na allocation (Fig. 5).
Scanning electron microscope-energy dispersive x-ray spectrometry (SEM–EDX)
Although the result of the above experiment does not exclude the possibility that light signals trigger Na influx into the leaf cells, it motivated us to test whether the starch granules have the capability of binding Na in V. riukiuensis. To do so, we performed SEM–EDX to locate Na in leaf sections (Fig. 6). We should note that the process of preparing leaf sections (including fixation) washes free Na+ ions away from vacuoles, cytoplasm and apoplastic spaces. Thus, SEM–EDX can detect only Na bound to cellular components.
As expected from the results of iodine–starch staining experiments (Fig. 3), the SEM image showed that there were few starch granules in the leaf sections of V. angularis whereas they were abundant in those of V. nakashimae and V. riukiuensis (Fig. 6).
The following EDX analysis detected few Na in the sections of V. angularis and V. nakashimae but detected Na in the sections of V. riukiuensis, especially in chloroplasts (Fig. 6). Moreover, Na was not in the middle of starch granules but was enriched around them (Fig. 6). As such, in the leaves of V. riukiuensis, Na was isolated in chloroplasts, especially around starch granules.
Effect of Na-binding on starch degradation
To elucidate if Na-bound starch granules were also degraded when shaded, we pre-treated the V. riukiuensis plants with 100 mM NaCl for 24 h, covered leaflets with aluminum foil for another 24 h and then conducted iodin-starch staining.
As a result, the shaded leaflets still exhibited significant staining (Fig. 7a), which highly contrasted from those without pre-treatment (Figs. 4, 5).
Na allocation in salt-acclimated plants of V. riukiuensis
As some plant species change patterns of Na allocation by salt acclimation (Noda et al. 2022), we also analyzed Na allocation in V. riukiuensis plants that were acclimated to salt stress. To do so, we pre-treated the plants with 50 mM NaCl without radioisotope for 3 days, fed 100 mM 22NaCl for another 3 days and then took autoradiographs.
As a result, the pattern of salt allocation of V. riukiuensis plants was similar to that of V. nakashimae (Figs. 1, 7b). Compared to the V. riukiuensis plant in Fig. 1, it allocated more Na to the root and the stems, and less to the leaves.
Tolerance of V. nakashimae and V. riukiuensis to acute salt stress
Because V. riukiuensis reduced Na allocation to the leaves when acclimated, we were intrigued to test a hypothesis that the Na capture by starch granules buffer impact of Na uptake before the plant adjusts itself to the high-salt condition. Thus, we treated the plants of V. nakashimae and V. riukiuensis with 200 mM NaCl without any pre-treatment with lower concentration of NaCl.
The plants of both species exhibited symptoms of etiolation for a few hours because of the sudden decrease of osmotic pressure, but they all recovered within another few hours. Though the plants looked recovered from the osmostress, the leaves of V. nakashimae exhibited severe injury in 2 days, while those of V. riukiuensis did not exhibit any visual damage (Fig. 7c).
Discussion
In this study, we demonstrated that V.riukiuensis accumulates starch granules that bind Na, which could be the second evidence of Na-binding starch granules in plants. The only report before this study was of common reed, which forms Na-binding starch granules in the shoot base in response to salt stress (Kanai et al. 2007). Kanai et al. have demonstrated co-localization of Na with starch granules and the isolated starch granules contained higher amounts of Na than other parts of the shoot base (Kanai et al. 2007). Although V. riukiuensis forms Na-binding starch granules in the leaves not in the shoot base (Figs. 1, 2, 3, 4, 5, 6), these facts suggest that the Na-trapping system by starch granules had independently evolved in multiple salt-tolerant species.
One may argue that our results in Fig. 5, where shading removed not only starch but Na allocation from the leaves, could be due to lack of light signals that may trigger Na influx into the leaf cells. Supporting such an argument, dark treatment suppressed the iron transport to the mesophyll cells in barley leaves (Bughio et al. 1997). We admit that our control experiment, where we covered the leaflets with cling films, excluded only the effect of transpiration on Na allocation to the leaves, but not the effect of light signals (Fig. 5). However, the previous report of Na-binding starch from common reed (Kanai et al. 2007) encouraged us to conduct SEM–EDX to directly test if the starch granules in V. riukiuensis are also able to bind Na. Fortunately, the SEM–EDX provided us the results that support our hypothesis (Fig. 6).
Since we previously revealed that V. riukiuensis allocates relatively higher amount of Na to the leaves, we have considered it has an includer-type mechanism, which sequesters excess Na+ into vacuoles (Noda et al. 2022; Yoshida et al. 2016). However, recent studies argue that the vacuole is not an ideal organelle for Na+ sequestration, as the tonoplast is permeable to Na+ and thus allows back-leak to cytosol (Shabala et al. 2020). Thus, for halophytic species including V. riukiuensis, it would be better to have a mechanism other than, or in addition to, the one using the vacuole. Na-binding starch granules could be one such mechanism, though we were surprised to see V. riukiuensis allocating Na to chloroplasts (Fig. 6), as Na is toxic especially to photosynthetic activity (Matoh et al. 1986). However, given Na bound to starch was not washed away during the process of sample fixation and section preparation (Fig. 6), it could serve to sequester and detoxify Na from cytosols and even within chloroplasts.
However, one can imagine that V. riukiuensis is sacrificing carbon storage for Na sequestration. As observed in Fig. 7a, starch degradation by shading, which is completely done within 24 h in the control condition, is not completed under salt stress. This result suggests that Na-bound starch granules are more difficult for a plant to use as energy source. Thus, if a plant had depended on starch granules for Na sequestration throughout its whole life, it would cause increased energy cost. However, reasonably, V. riukiuensis stops allocating Na to the leaves when it is acclimated to salt stress. As shown in Fig. 7b, preculture with 50 mM NaCl for 3 days enhanced Na allocation to the root and the stems, which is more like V. nakashimae (Fig. 1).
If V. riukiuensis also has an ability to suppress Na allocation to the leaves, what is the merit of having Na-binding starch? We consider it could buffer Na toxicity at the initiation of salt stress. As also observed in V. nakashimae in our previous study (Noda et al. 2022), acclimation enhanced Na evacuation in the root and the stem (Fig. 7b). Thus, before acclimation is complete, Na evacuation by the root and the stem is not strong enough to suppress Na allocation to the leaves. However, Na flown into leaf cells can be trapped and detoxified with Na-binding starch granules. The tolerance of V. riukiuensis in acute salt stress might reflect such buffering effect of Na-binding starch (Fig. 7c).
This result also indicates that Na-binding activity is characteristic to the starch granules in V. riukiuensis and not to those of V. nakashimae. It was first complicating that V. nakashimae also accumulates lots of starch granules in the chloroplasts but does not allocate Na to the leaves. It is still possible that V. nakashimae has an exceptional ability to completely exclude Na+ out of the leaves or that its chloroplasts simply do not incorporate lots of Na, but the sensitivity to the acute stress (Fig. 7c) does not support these ideas. Thus, the starch granules in V. nakashimae probably lack Na-binding ability.
Although pure amylose chains do not have any ability to bind cations including Na+, modification of hydroxyl groups such as phosphorylation turns them into cation exchangers (Matsumoto et al. 1998). Given amylose chains are extended from the surface of starch granules (Goren et al. 2018), V. riukiuensis and common reed may have higher enzymatic activity in modifying those chains. In addition, other studies also report that α-glucan-like molecules in common reeds bind cadmium (Cd) ions and reduce Cd stress (Higuchi et al. 2013, 2015). As such, some plant species have acquired abilities to produce and accumulate ion exchangers to attenuate sodium and other metal ion stresses.
Lastly, we mention the environmental difference between the habitats of V. riukiuensis and V. nakashimae, which may explain the importance of Na-binding starch in V. riukiuensis. Though both species live in coasts of remote islands, those of V. riukiuensis are located in the tropics (Iseki et al. 2016) and are frequently hit by typhoons. Thus, compared to V. nakashimae, whose habitat is located in temperate areas (Iseki et al. 2016), V. riukiuensis is often exposed to acute salt stress by high tide and sea spray. Such an environment might have selected the higher tolerance of V. riukiuensis, to which Na-binding starch could contribute.
To conclude, we have demonstrated common reed is not the only species to have evolved Na-binding starch granules. As it is effective in multiple plant taxa, we are intrigued to apply this system for developing salt-tolerant crops. By combining other mechanisms of salt tolerance, it would bring synergistic effects on salt tolerance.
Data availability
Data are available on request from authors.
References
Bughio N, Takahashi M, Yoshimura E, Nishizawa KN, Mori S (1997) Light-dependent iron transport into isolated barley chloroplasts. Plant Cell Physiol 38:101–105
Goren A, Ashlock D, Tetlow IJ (2018) Starch formation inside plastids of higher plants. Protoplasma 255:1855–1876
Higuchi K, Tsuchiya M, Nakata S, Tanabe A, Fukawa S, Kanai M, Miwa E (2013) Detoxification of cadmium (Cd) by a novel Cd-associated and Cd-induced molecule in the stem of common reed. J Plant Physiol 170:1553–1560
Higuchi K, Kanai M, Tsuchiya M, Ishii H, Shibuya N, Fujita N, Nakamura Y, Suzui N, Fujimaki S, Miwa E (2015) Common reed accumulates starch in its stem by metabolic adaptation under Cd stress conditions. Front Plant Sci 6:138
Iseki K, Takahashi Y, Muto C, Naito K, Tomooka N (2016) Diversity and evolution of salt tolerance in the genus Vigna. PLoS ONE 11:e0164711
Kanai M, Higuchi K, Hagihara T, Konishi T, Ishii T, Fujita N, Nakamura Y, Maeda Y, Tadano YM (2007) Common reed produces starch granules at the shoot base in response to salt stress. New Phytol 176:572–580
Matoh T, Kairusmee P, Takahashi E (1986) Salt-induced damage to rice plants and alleviation effect of silicate. Soil Sci Plant Nutr 32:295–304
Matsumoto K, Hirayama C, Motozato Y (1998) Preparation of bead-shaped starch ion exchangers and their properties. Nippon Kagaku Kaishi 10:657–663
Noda Y, Sugita R, Hirose A, Kawachi N, Tanoi K, Furukawa J, Naito K (2022) Diversity of Na+ allocation in salt-tolerant species of the genus Vigna. Breed Sci 72:326–331
Panta S, Flowers T, Lane P, Doyle R, Shabala HG (2014) Halophyte agriculture: success stories. Environ Exp Bot 107:71–83
Shabala S, Chen G, Chen ZH, Pottosin I (2020) The energy cost of the tonoplast futile sodium leak. New Phytol 225:1105–1110
Tomooka N, Naito K, Kaga A et al (2014) Evolution, domestication and neo-domestication of the genus Vigna. Plant Genetic Resour 12:168–171
van Zonneveld M, Rakha M, Tan SY et al (2020) Mapping patterns of abiotic and biotic stress resilience uncovers conservation gaps and breeding potential of Vigna wild relatives. Sci Rep 10:2111
Wada Y, van Beek LPH, van Kempen CM et al (2010) Global depletion of groundwater resources. Geophys Res Lett 37:L20402
Yoshida Y, Marubodee R, Ogiso-Tanaka E et al (2016) Salt tolerance in wild relatives of adzuki bean, Vigna angularis (Willd.) Ohwi et Ohashi. Genet Resour Crop Evol 63:627–637
Funding
This study was financially supported by JSPS KAKENHI Grant Number 18H02182, 19KK0148, 20H02885, JST PRESTO Grant Number 11103610, Moonshot R&D Program for Agriculture, Forestry and Fisheries by Cabinet Office, Government of Japan (JPJ009237), Environmental Radioactivity Research Network Center (Y-19-05) and Interdisciplinary Project on Environmental Transfer of Radionuclides (No. Y-1).
Author information
Authors and Affiliations
Contributions
KN conceived the study. YN, AH, KT, NK, JF, KT and KN designed experiments. YN, AH, MA, MS, MW and KT performed experiments. YN, AH, MS and KN analyzed data. AH, MS, KT and JF tested the results. YN and KN wrote the paper.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflicts of interest to declare that are relevant to the content of this article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Noda, Y., Hirose, A., Wakazaki, M. et al. Starch-dependent sodium accumulation in the leaves of Vigna riukiuensis. J Plant Res 136, 705–714 (2023). https://doi.org/10.1007/s10265-023-01470-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10265-023-01470-8