Abstract
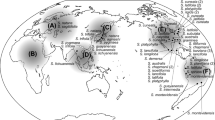
Ottelia, a pantropical genus of aquatic plants belonging to the family Hydrocharitaceae, includes several narrowly distributed taxa in Asia. Although the Asian species have received comparatively more research attention than congeners in other areas, various key taxonomic questions remain unaddressed, especially with regards to apparent cryptic diversity within O. alismoides, a widespread species complex native to Asia, northern Australia and tropical Africa. Here we test taxonomic concepts and evaluate species boundaries using a phylogenetic framework. We sampled five of the seven species of Ottelia in Asia as well as each species endemic to Africa and Australia; multiple samples of O. alismoides were obtained from across Asia. Phylogenetic trees based on five plastid DNA markers and the nuclear ITS region shared almost identical topologies. A Bayesian coalescent method of species delimitation using the multi-locus data set discerned one species in Africa, one in Australia and four in Asia with the highest probability. The results lead us to infer that a population sampled in Thailand represents a hitherto unrecognised cryptic taxon within the widespread species complex, although the apparent lack of unambiguous diagnostic characters currently precludes formal description. Conversely, no molecular evidence for distinguishing O. cordata and O. emersa was obtained, and so the latter is synonymised under the former. Two accessions that exhibit inconsistent positions among our phylogenetic trees may represent cases of chloroplast capture, however incomplete lineage sorting or polyploidy are alternative hypotheses that ought to be tested using other molecular markers.



Similar content being viewed by others
References
Angulo A, Icochea J (2010) Cryptic species complexes, widespread species and conservation: lessons from Amazonian frogs of the Leptodactylus marmoratus group (Anura: Leptodactylidae). Syst Biodiv 8:357–370
Baldwin BG (1992) Phylogenetic utility of the internal transcribed spacers of nuclear ribosomal DNA in plants: an example from the Compositae. Mol Phylogenet Evol 1:3–16
Bouckaert RR, Heled J, Kühnert D, Vaughan TG, Wu C-H, Xie D, Suchard MA, Rambaut A, Drummond AJ (2014) BEAST 2: a software platform for Bayesian evolutionary analysis. PLoS Comput Biol 10:e1003537
Chase MW, Cowan RS, Hollingsworth PM, van den Berg C, Madriñan S, Petersen G, Seberg O, Jørgsensen T, Cameron KM, Carine M, Pedersen N, Hedderson TAJ, Conrad F, Salazar GA, Richardson JE, Hollingsworth ML, Barraclough TE, Kelly L, Wilkinson M (2007) A proposal for a standardised protocol to barcode all land plants. Taxon 56:295–299
Chen LY, Chen JM, Gituru RW, Wang QF (2012) Generic phylogeny, historical biogeography and character evolution of the cosmopolitan aquatic plant family Hydrocharitaceae. BMC Evol Biol 12:30
Cook CDK (1996) Aquatic and wetland plants of India. Oxford University, Oxford
Cook CDK, Urmi-König K (1984) A revision of the genus Ottelia (Hydrocharitaceae). 2. The species of Eurasia, Australasia and America. Aquat Bot 20:131–177
Cook CDK, Symoens J-J, Urmi-König K (1983) A revision of the genus Ottelia (Hydrocharitaceae). 1. Generic considerations. Aquat Bot 18:263–274
Degnan JH, Rosenberg NA (2009) Gene tree discordance, phylogenetic inference and the multispecies coalescent. Trends Ecol Evol 24:332–340
den Hartog C (1957) Hydrocharitaceae. In: van Steenis CGGJ (ed) Flora Malesiana, ser. 1, vol 5. Noordhoff-Kollf. Djakarta, pp 381 − 413
Drummond AJ, Rambaut A (2007) BEAST: bayesian evolutionary analysis by sampling trees. BMC Evol Biol 7:214–222
Drummond AJ, Ho SYW, Phillips MJ, Rambaut A (2006) Relaxed phylogenetics and dating with confidence. PLoS Biol 4:e88
Felsenstein J (1985) Confidence limits on phylogenies: an approach using the bootstrap. Evolution 39:783–791
Funk WC, Caminer M, Ron SR (2012) High levels of cryptic species diversity uncovered in Amazonian frogs. Proc R Soc B 279:1806–1814
Govaerts R (2018) World checklist of Hydrocharitaceae. Facilitated by the royal botanic gardens, Kew. Published online at http://apps.kew.org/wcsp/. Retrieved 22 December 2018
Haynes RR (2001) Hydrocharitaceae. In: Santisuk T, Larsen K (eds) Flora of Thailand, vol 7. The Forest Herbarium. Royal Forest Department, Bangkok, pp 365–382
He J-B, Sun X-Z (1990) Ottelia emersa Z.C. Zhao et R.L. Luo (Hydrocharitaceae)—a Synonym of O. cordata (Wallich) Dandy. Aquat Bot 36:395–398
He J-B, Sun X-Z, Wang H-Q (1990) Taxonomy of the bisexual species of Ottelia in China. Aquat Bot 36:389–393
Hutchinson J, Dalziel JM (1958) Flora of west tropical Africa vol. I. part 2 (2nd edn revised by Keay RWJ). Royal Botanic Gardens, Kew, pp 1662–1664
Ito Y, Ohi-Toma T, Murata J, Tanaka N (2010) Hybridization and polyploidy of an aquatic plant, Ruppia (Ruppiaceae), inferred from plastid and nuclear DNA phylogenies. Am J Bot 97:1156–1167
Ito Y, Tanaka N, Albach DC, Barfod AS, Oxelman B, Muasya AM (2017) Molecular phylogeny of the cosmopolitan aquatic plant genus Limosella (Scrophulariaceae) with a particular focus on the origin of the Australasian L. curdieana. J Plant Res 140:107–116
Jacobs SWL, McColl KA (2011) Hydrocharitaceae. In: Wilson AJG (ed) Flora of Australia, vol 39. ABRS/CSIRO, Melbourne, pp 14–44
Johnson LA, Soltis DE (1998) Assessing congruence: empirical examples from molecular data. In: Soltis DE, Soltis PS, Doyle JJ (eds) Molecular systematic of plants II: DNA sequencing. Kluwer Academic Publisher, Norwell, pp 265–296
Jones GL (2017) Algorithmic improvements to species delimitation and phylogeny estimation under the multispecies coalescent. J Math Biol 74:447–467
Jones GL, Aydin Z, Oxelman B (2015) DISSECT: an assignment-free Bayesian discovery method for species delimitation under the multispecies coalescent. Bioinformatics 31:991–998
Juffe-Bignoli, D (2011) Ottelia cordata. The IUCN red list of threatened species 2011: e.T194035A8879309. http://dx.doi.org/10.2305/IUCN.UK.2011-2.RLTS.T194035A8879309.en. Downloaded on 09 January 2019
Katoh K, Standley DM (2013) MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Mol Biol Evol 30:772–780
Les DH, Tippery NP (2013) In time and with water. the systematics of alismatid monocotyledons. In: Wilkin P, Mayo SJ (eds) Early events in monocot evolution. Cambridge University Press, Cambridge, pp 119–164
Les DH, Cleland MA, Waycott M (1997) Phylogenetic studies in Alismatidae, II: evolution of marine angiosperms (‘seagrasses’) and hydrophily. Syst Bot 22:443–463
Les DH, Moody ML, Soros C (2006) A reappraisal of phylogenetic relationships in the monocotyledon family Hydrocharitaceae. In: Columbus JT, Friar EA, Porter JM, Prince LM, Simpson MG (eds) Monocots: comparative biology and evolution: excluding Poales. Rancho Santa Ana Botanical Garden, Claremont, pp 211–230
Little DP, Barrington DS (2003) Major evolutionary events in the origin and diversification of the fern genus Polystichum (Dryopteridaceae). Am J Bot 90:508–514
Lohman DJ, Ingram KK, Prawiradilaga DM, Winker K, Sheldon FH, Moyle RG, Ng PKL, Ong PS, Wang LK, Braile TM, Astuti D, Meier R (2010) Cryptic diversity in “widespread” southeast Asian bird species suggests that Philippine avian endemism is gravely underestimated. Biol Conserv 143:1885–1890
Luo RL, Wang HQ (1987) A new species of Ottelia (Hydrocharitaceae) and its karyotype. J Wuhan Bot Res 5:339–342
Manthey JD, Klicka J, Spellman GM (2011) Cryptic diversity in a widespread North American songbird: phylogeography of the Brown Creeper (Certhia americana). Mol Phylogenet Evol 58:502–512
McNeill J, Barrie FR, Buck WR, Demoulin V, Greuter W, Hawksworth DL, Herendeen PS, Knapp S, Marhold K, Prado J, Prud’homme Van Reine WF, Smith GF, Wiersema JH, Turland NJ (eds) (2012) International code of nomenclature for algae, fungi and plants (Melbourne Code): Adopted by the Eighteenth International Botanical Congress, Melbourne, Australia, July 2011. Regnum Vegetabile 154. Koeltz Scientific Books, Königstein
Miller MA, Pfeiffer W, Schwartz T (2010) Creating the CIPRES Science Gateway for inference of large phylogenetic trees. In: Proceedings of the gateway computing environments workshop (GCE), 14 Nov. 2010, New Orleans, pp 1–8. http://www.phylo.org/sub_sections/portal/cite.php
Nylander JAA (2002) MrModeltest. Ver. 1.0. Program distributed by the author. Department of Systematic Zoology, Uppsala University, Uppsala. Retrieved from http://www.ebc.uu.se/systzoo/staff/nylander.html
Oliver PM, Adams M, Lee MSY, Hutchinson MN, Doughty P (2009) Cryptic diversity in vertebrates: molecular data double estimates of species diversity in a radiation of Australian lizards (Diplodactylus, Gekkota). Proc R Soc B 276:2001–2007
Olmstead RG, Sweere JA (1994) Combining data in phylogenetic systematics: an empirical approach using three molecular data sets in the Solanaceae. Syst Biol 43:467–481
Oxelman B, Backlund M, Bremer B (1999) Relationships of the Buddlejaceae s. l. inferred from chloroplast rbcL and ndhF sequences. Syst Bot 24:164–182
Phiri EE, Daniels SR (2016) Multilocus coalescent species delimitation reveals widespread cryptic differentiation among Drakensberg mountain-living freshwater crabs (Decapoda: Potamonautes). Invertebr Syst 30:60–74
Rambaut A (2009) FigTree ver. 1.3.1: Tree Figure Drawing Tool. Retrieved from http://tree.bio.ed.ac.uk/software/figtree/
Rambaut A, Suchard MA, Xie W, Drummond AJ (2014) Tracer. ver. 1.6. Retrieved from http://beast.bio.ed.ac.uk/Tracer
Ronquist F, Huelsenbeck JP (2003) MrBayes 3: bayesian phylogenetic inference under mixed models. Bioinformatics 19:1572–1574
Ronquist F, Teslenko M, van der Mark P, Ayres DL, Darling A, Höhna S, Larget B, Liu L, Suchard MA, Huelsenbeck JP (2012) MrBayes 3.2: efficient Bayesian phylogenetic inference and model choice across a large model space. Syst Biol 61:539–542
Shimodaira H (2002) An approximately unbiased test of phylogenetic tree selection. Syst Biol 51:492–508
Stamatakis A, Hoover P, Rougemont J (2008) A rapid bootstrap algorithm for the RAxML web servers. Syst Biol 57:758–771
Swofford DL (2002) PAUP: Phylogenetic analysis using parsimony (and other methods). ver. 40b10. Sinauer Associates, Sunderland
Tanaka N (2015) Hydrocharitaceae. In: Ohashi H, Kadota Y, Murata J, Yonekura K, Kihara H (eds) Wild flowers of Japan, vol 1. Heibonsha, Tokyo, pp 118–125 (in Japanese)
Tanaka N, Setoguchi H, Murata J (1997) Phylogeny of the family Hydrocharitaceae inferred from rbcL and matK gene sequence data. J Plant Res 110:329–337
Tippery NP, Les DH (2011) Evidence for the hybrid origin of Nymphoides montana Aston (Menyanthaceae). Telopea 13:285–294
Wang QF, Guo YH, Haynes RR, Hellquist CB (2010) Hydrocharitaceae. In: Wu C-Y, Raven PH, Hong D-Y (eds) Flora of China, vol 23. Science Press. Beijing & Missouri Botanical Garden Press, St. Louis, pp 91–102
Wendel JF, Doyle JJ (1998) Phylogenetic incongruence: window into genome history and molecular evolution. In: Soltis PS, Soltis DE, Doyle JJ (eds) Molecular systematics of plants II. Kluwer Academic Publishing, Boston, pp 265–296
Wolf PG, Soltis PS, Soltis DE (1994) Phylogenetic relationships of Dennstaedtioid ferns: evidence from rbcL sequences. Mol Phylogenet Evol 3:383–392
Yang Z, Rannala B (1997) Bayesian phylogenetic inference using DNA sequences: a Markov Chain Monte Carlo method. Mol Biol Evol 14:717–724
Acknowledgements
The authors thank H. Wang (HITBC) and E. Liu (KUN) for arranging loans from their institutions and/or for hospitality during the authors’ recent visits; R. Pooma, S. Saengrit, N. Suphuntee, K. Chayamarit (BKF), K. Shuto (Fukushima), S.R. Yadav (Shivaji), H. Murata (Osaka), T. Sugawara (MAK), Nb. Tanaka (TNS), J. Murata, T. Ohi-Toma (TI), A. Naiki, Y. Saito (Okinawa) for assistance in the field; and C. Ishii (Tsukuba) for help with DNA sequencing. This research was supported by Chinese Academy of Sciences (CAS) President’s International Fellowship Initiative (PIFI) Grant no. 2015PB022 to YI and by the ‘Integrated analysis of natural history collections for conservation of highly endangered species’ programme under the National Museum of Nature and Science, Japan to NT.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ito, Y., Tanaka, N., Barfod, A.S. et al. Molecular phylogenetic species delimitation in the aquatic genus Ottelia (Hydrocharitaceae) reveals cryptic diversity within a widespread species. J Plant Res 132, 335–344 (2019). https://doi.org/10.1007/s10265-019-01109-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10265-019-01109-7