Abstract
Chronic kidney disease (CKD) represents a significant global public health issue, with its incidence and prevalence escalating annually. Metabolic disorders are one of the major etiological factors of CKD. This study investigates the relationship between the emerging metabolic index triglyceride-glucose body mass index (TyG-BMI) and the onset of CKD. Our study enrolled 3,485 healthy participants (1,576 men and 1,909 women), with a follow-up period of 3 years. The primary outcome was the emergence of CKD, defined by an eGFR less than 60 mL/(min × 1.73 m2) or the onset of proteinuria. To examine the TyG-BMI and CKD onset relationship, we used univariate and multivariate logistic regression analyses, stratified analyses, and receiver operating characteristic (ROC) curves. After a three-year follow-up, CKD developed in 2% (n = 70) of the participants. Subjects were divided into three equal groups based on their TyG-BMI values, from lowest to highest. After adjusting for potential confounders, the highest TyG-BMI group exhibited a multifactor-adjusted odds ratio (OR) of 4.24 (95% CI 1.30–13.78, P = 0.016) compared to the lowest group. Stratified analyses revealed that the association between TyG-BMI and CKD onset was stronger among females, individuals younger than 60 years, and those with a BMI ≥ 24 kg/m2. Furthermore, TYG-BMI was effective in predicting the incidence of CKD. Our findings indicate that TyG-BMI is an independent risk factor for the onset of CKD and that assessment of TyG-BMI may be useful for the early identification of individuals at high risk for CKD.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Chronic kidney disease (CKD) is characterised by structural or functional abnormalities of the kidneys caused by a variety of factors, often with or without a reduction in glomerular filtration rate (GFR) [1, 2]. Currently, the incidence and prevalence of CKD are increasing annually, and it is predicted to become the fifth leading cause of death worldwide by 2040 [3]. In its early stages, CKD may have no or only mild symptoms, including fatigue, nausea and nocturia [4, 5]. As the disease progresses, symptoms worsen, leading to serious complications such as high blood pressure, heart failure, electrolyte and central nervous system disorders, and potentially life-threatening conditions in the kidney failure stage [4, 5]. Traditional methods of diagnosing CKD, such as serum creatinine (SCr) and estimated glomerular filtration rate (eGFR), have significant limitations and often fail to detect the disease early, and many patients are not diagnosed until they have reached stage three (30 ml/(min/1.73 m2) ≤ eGFR < 60 ml/(min/1.73 m2)) [6]. Therefore, early detection and intervention in patients with CKD can delay disease progression and reduce the incidence of kidney failure.
Growing research suggests metabolic syndrome is a key risk factor for CKD [7]. First identified by Reaven in 1988 as "insulin resistance syndrome", metabolic syndrome includes insulin resistance, impaired glucose tolerance, abdominal obesity, high triglyceride levels, low HDL cholesterol and hypertension [8]. Insulin resistance is a typical feature of the metabolic syndrome, and the current gold standard for assessing IR is the hyperinsulinic normoglycaemic clamp, which is not suitable for clinical use because it is invasive and expensive [9]. HOMA-IR, another measure of insulin resistance, requires multiple blood samples and is less suitable for patients with impaired β-cell function or on insulin therapy, limiting its clinical applicability [10]. The triglyceride-glucose (TyG) index, an emerging IR indicator, is similar to the gold standard clamp test, outperforms HOMA-IR in detecting IR in the general population, and is easily accessible in clinical settings [11, 12]. More importantly, recent studies have shown that the TyG index in combination with obesity parameters (BMI, waist circumference and WHtR) can significantly improve the validity of the assessment [13]. For instance, the TyG-BMI index, Ln [TG (mg/dl) × FBG (mg/dl)/2] × BMI (kg/m2), incorporates key markers of the metabolic syndrome (triglycerides, fasting glucose, BMI) and is closely associated with the incidence or prognosis of metabolic diseases such as hypertension, diabetes mellitus, stroke, coronary heart disease, heart failure and non-alcoholic fatty liver disease [14, 15]. TyG has been identified as a predictor of CKD and serves as a risk stratification tool for CKD in individuals at high risk with cardiovascular comorbidities, but the specific relationship between TyG-BMI and CKD has not been reported [16].
In the prospective study collecting data from a substantial cohort with baseline eGFR ≥ 60 mL/min/1.73 m2 and no proteinuria, we aimed to investigate the TyG-BMI's correlation with CKD onset, offering insights into early screening, diagnosis, and self-monitoring for CKD in high-risk populations.
Materials and methods
Ethical statement
All research activities were conducted in accordance with the guidelines of the Medical Ethics Committee of 2013 and the Declaration of Helsinki, and received approval from the Medical Ethics Committee of Tongji Medical College, Huazhong University of Science and Technology. Written informed consent was obtained from all participants before their inclusion in the study.
Study participants
We collected data from 3,761 participants who attended health examinations at Wuhan Union Hospital during 2015–2016, and rigorously screened them according to inclusion and exclusion criteria. The final sample consisted of 3,485 participants, including 1,576 males (45.22%) and 1,909 females (54.78%). Data were collected via questionnaires and electronic medical record systems, and participants were followed up for a period of 3 years.
Inclusion and exclusion criteria
Inclusion criteria were as follows: Participants collected were all older than 18 years of age, with complete baseline data such as fasting blood glucose, BMI, triglycerides, kidney function tests, urinalysis, renal ultrasound, and medical history, and were able to complete the three-year follow-up.
Exclusion criteria were as follows: Participants with chronic kidney disease (CKD) at the start of the study, defined as an eGFR < 60 ml/(min/1.73 m2) and/or positive urinary protein, were excluded. Also excluded were individuals with serious renal diseases such as congenital anomalies of the kidney and urinary tract, history of renal surgery (kidney transplant, nephrectomy), renal atrophy, or sponge kidney. Additionally, participants who were missing data on eGFR, urinary protein, fasting blood glucose, triglycerides, or BMI at the end of the three-year follow-up were also excluded.
Measurements and outcomes
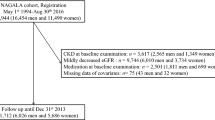
Standardized physical examination procedures were performed on the participants, and relevant data were collected by trained physicians. This included general information such as name, age, gender, examination ID, marital status, educational background, smoking status, alcohol consumption, physical activity, and employer; medical history including hypertension, coronary artery disease, diabetes, and history of renal surgery; physical examination data such as heart rate, pulse, systolic and diastolic blood pressure, height, and weight (BMI was calculated as weight in kilograms divided by the square of height in meters); biochemical indicators including fasting blood glucose, total cholesterol, triglycerides, low-density lipoprotein cholesterol, high-density lipoprotein cholesterol, serum creatinine, blood urea nitrogen, estimated glomerular filtration rate (eGFR), uric acid, alanine transaminase, and aspartate transaminase (Fig. 1).
Calculation of the TYG-BMI index and diagnostic criteria for new-onset CKD
The formula for calculating the TYG-BMI index is Ln [1/2 fasting plasma glucose (mg/dL) × fasting triglycerides (mg/dL)] × BMI.
The diagnostic criteria for CKD are based on the recommendations of the Kidney Disease: Improving Global Outcomes (KDIGO) 2021, namely an eGFR value below 60 ml/(min/1.73 m2) and/or the new occurrence of proteinuria [17]. The eGFR calculation follows the formula recommended by the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI): eGFR = a × (SCr / b) ^c × (0.993) ^age, where for the female population a = 144, b = 0.7, c = − 0.329 (SCr ≤ 0.7 mg/dL) or − 1.209 (SCr > 0.7 mg/dL); for the male population a = 141, b = 0.9, c = − 0.411 (SCr ≤ 0.9 mg/dL) or − 1.209 (SCr > 0.9 mg/dL).
Statistical analysis
All statistical analyses in this investigation were executed using R software, version 3.3.0. Data were consistently quantified and coded. In Table 1, participants' TYG-BMI indices were arranged in ascending order and segmented into tertiles. Baseline characteristics for continuous variables were denoted as mean ± standard error, and categorical variables were denoted as frequency (%). The normality of the data was assessed using the Kolmogorov–Smirnov test. Normally distributed variables were compared using one-way ANOVA among multiple groups. Non-normally distributed variables were represented by medians (M) and analyzed using rank-sum tests. Categorical data comparisons utilized the χ2 test. Univariate logistic regression was applied in Fig. 2 to examine the relationship between TYG-BMI and other risk factors for CKD incidence. Table 2 presents the results of binary logistic regression analysis to determine whether TYG-BMI is an independent risk factor for CKD. Four models were constructed: An uncorrected model (without adjustment for any variables), Model 1 (adjusted for age, sex, BMI, systolic and diastolic blood pressure, fasting blood glucose, total, HDL and non-HDL cholesterol, and triglycerides), Model 2 (building on Model 1 with further adjustment for alanine aminotransferase, aspartate aminotransferase, alkaline phosphatase, glutathione, serum albumin, serum glucose, and serum albumin), and Model 3 (extending Model 2 with adjustments for coronary heart disease and diabetes mellitus). Figure 3 assess the association between TyG-BMI index and the risk of new-onset CKD in different demographic groups, stratified analyses based on sex, age and BMI were performed using multifactorial logistic regression within each tertile. The ROC analysis in Fig. 4 calculated the AUC to demonstrate the predictive ability of TyG-BMI for the incident of CKD. All tests were two-tailed, with P < 0.05 indicating statistical significance.
Logistic regression lines for univariate analyses are shown in dark blue, and 95% confidence intervals are shown in light blue. p values are for univariate logistic regression. A–I indicate the relationship between the probability of CKD and TyG-BMI, TyG, TG (triglycerides), GLU (fasting glucose), LDL (low-density lipoprotein cholesterol), albumin, SBP (systolic blood pressure), BUN (blood urea nitrogen levels), and eGFR (estimated glomerular filtration rate), respectively (colour figure online)
Results
Baseline information
A total of 3,761 records were collected from individuals who underwent physical examinations at Wuhan Union Hospital between 2015 and 2016. After excluding 133 records due to missing data on baseline eGFR values, urinary protein, fasting blood glucose, BMI, or triglycerides, 3,628 participants were included for screening. An additional 131 cases were excluded due to CKD (eGFR values less than 60 ml/(min/1.73 m2) or proteinuria), previous history or renal ultrasound findings of severe renal diseases (including congenital dysplasia of both kidneys, renal transplantation, unilateral or bilateral nephrectomy, renal atrophy, and sponge kidney), and 12 cases with incomplete follow-up data (eGFR values or urine protein). The final cohort for statistical analysis comprised 3,485 records. By the end of the follow-up, a total of 70 participants had developed CKD, giving a cumulative prevalence of 2% (Fig. 1).
Data from 3,485 individuals were categorized into three groups based on the TyG-BMI index, arranged from lowest to highest. The TyG-BMI indices were as follows: Group 1 ranged from 115.97 to 172.01, Group 2 from 172.02 to 206.45, and Group 3 from 206.46 to 444.46. Upon comparing and analyzing the baseline information of the three groups, we found the incidence of new-onset CKD progressively increased with higher TyG-BMI groups. Additionally, as the TyG-BMI group increased, there was a significant increase in age, BMI, systolic and diastolic blood pressure, fasting blood glucose, ALT, AST, ALP, GGT, urea nitrogen, creatinine, uric acid, total cholesterol, LDL cholesterol, and triglycerides. Similarly, the prevalence of pre-existing hypertension, coronary heart disease, and diabetes mellitus among participants increased. Conversely, HDL cholesterol levels decreased. All observed differences were statistically significant. Additionally, there were no statistically significant differences in albumin levels among the three groups (Table 1).
Screening for risk factors influence new-onset CKD
To elucidate the relationships between the factors listed in Table 1 and the incidence of new-onset CKD, unrestricted cubic splines were employed for flexible modeling, allowing for the visualization and prediction of these associations. The analysis revealed positive correlations between TyG-BMI, TyG, triglycerides, fasting glucose, systolic blood pressure, and blood urea nitrogen levels with the onset of CKD (P < 0.05), indicating that an increase in indicators such as TyG-BMI was associated with a higher likelihood of developing CKD; on the contrary, there was a negative correlation between low-density lipoprotein cholesterol, serum albumin, estimated glomerular filtration rate, and new-onset CKD (P < 0.05), i.e., as estimated glomerular filtration rate increased, the corresponding incidence of CKD decreased (Fig. 2).
Risk association between TyG-BMI and new-onset CKD
To reduce the effect of confounding factors, we performed four logistic regression models: An uncorrected model (without adjustment for any variables), Model 1 (adjusted for age, sex, BMI, systolic and diastolic blood pressure, fasting blood glucose, total, HDL and non-HDL cholesterol, and triglycerides), Model 2 (building on Model 1 with further adjustment for alanine aminotransferase, aspartate aminotransferase, alkaline phosphatase, glutathione, serum albumin, serum glucose, and serum albumin), alanine aminotransferase, aspartate aminotransferase, alkaline phosphatase, glutamyl transpeptidase, albumin, serum creatinine, blood urea nitrogen, and blood uric acid) and Model 3 (extending Model 2 with adjustments for coronary heart disease and diabetes mellitus). The results showed an odds ratio (OR) of 2.83 (95% CI 1.49–5.36, P = 0.001) for the third group in the uncorrected model, 4.05 (95% CI 2.26–8.27, P < 0.001) in Model 1, 4.52 (95% CI 1.39–14.66, P = 0. 012) in Model 2, and 4.24 (95% CI 1.30–13.78, P = 0.016) in Model 3, suggesting that high TyG-BMI was an independent risk factor for new-onset CKD (Table 2).
Stratified analysis by sex, age, and BMI
To assess the association between TyG-BMI index and the risk of new-onset CKD in different demographic groups, stratified analyses based on sex, age and BMI were performed using multifactorial logistic regression within each subgroup. Sex-stratified analysis showed a more significant association between the TyG-BMI index and incident CKD in women, with a corrected OR of 1.15 (95% CI 1.01–1.30, P = 0.0341) compared with 1.00 (95% CI 0.94–1.06, P = 0.914) in men, although the difference was not statistically significant. Age-stratified analysis showed a stronger association in those younger than 60 years, with a corrected OR of 1.14 (95% CI 1.02–1.28, P = 0.021) compared with 1.00 (95% CI 0.62–1.02, P = 0.742) in those older than 60 years, although this difference was also not statistically significant. In the BMI-stratified analysis, a stronger association was observed in those with a BMI ≥ 24 kg/m2, with a corrected OR of 1.14 (95% CI 1.02–1.28, P = 0.025) compared with 1.01 (95% CI 0.95–1.08, P = 0.697) in those with a BMI < 24 kg/m2, although the difference was not statistically significant (Fig. 3).
Ability of TyG-BMI to predict new-onset CKD
To assess the predictive capability of the TyG-BMI index for new-onset CKD, the receiver operating characteristic (ROC) curve was plotted, and the area under the curve (AUC) was calculated. The AUC was 0.857, suggesting that the TyG-BMI index possesses a strong predictive ability for new-onset CKD (Fig. 4).
Discussion
In this prospective study, we found that elevated TyG-BMI was an independent risk factor for incident CKD and was effective in predicting incident CKD. In addition, the association between TyG-BMI and incident CKD was significantly stronger in women, those younger than 60 years, and those with a body mass index ≥ 24 kg/m2.
CKD is a significant global public health challenge due to its complex and diverse etiology [18]. Early diagnosis of CKD is hindered by the limitations of commonly used clinical diagnostic methods, such as serum creatinine or eGFR, and many patients are diagnosed in the mid to late stages of the disease [6, 19]. Hence, investigating the indicators for early clinical diagnosis of CKD is of paramount importance. In recent years, metabolic diseases have emerged as important risk factors for CKD [20]. Critical indicators associated with metabolic diseases, such as insulin resistance, hyperglycemia, dyslipidemia, and obesity, have been shown to play a significant role in the development of CKD [20]. The TyG-BMI index, an emerging metric for assessing insulin resistance levels, ingeniously combines triglyceride levels, blood glucose, and body mass index [21]. Research has shown that TyG-BMI is an independent risk factor for metabolic diseases, including diabetes and stroke [22,23,24]. Jiang et al. have identified the TyG-BMI as a significant risk factor for diabetic kidney disease (DKD) in patients with newly diagnosed type 2 diabetes (T2D). Their research demonstrates that elevated TyG-BMI levels are correlated with an increased risk of developing DKD. The study highlights a dose–response relationship, indicating a linear correlation between TyG-BMI and DKD risk. Furthermore, the findings suggest a potential association between the TyG-BMI index and chronic kidney disease (CKD) [25].
Our study supports TyG-BMI as an independent risk factor for incident CKD and confirms its efficacy in predicting incident CKD. Additionally, there was a significant correlation between the TyG-BMI index and incident CKD, which persisted even after rigorous data screening based on inclusion and exclusion criteria and adjustment for common CKD risk factors, such as age, sex, BMI, hypertension, lipids, serum creatinine, blood urea nitrogen, blood uric acid, coronary heart disease, and diabetes mellitus. Therefore, the TyG-BMI index is critical for early screening of high-risk CKD groups.
This study also performed stratified analyses to examine the association between the TyG-BMI index and incident CKD by sex, age and BMI. The literature indicates that the incidence of CKD is significantly higher in women than in men, possibly due to the longer life expectancy of women, which may lead to possible inaccuracies in the assessment of CKD using eGFR [26, 27]. However, different studies reach different conclusions when the characteristics of the study population are changed. Other evidence suggests that disease progression is faster in men than in women with CKD, possibly due to the protective effects of estrogen on the kidneys and the detrimental effects of testosterone in men [28, 29]. In our study, the association between TyG-BMI and incident CKD was stronger in women (OR 1.15; 95% CI 1.01–1.30; P = 0.034) than in men, which may be related to the fact that the subjects in our study were predominantly older than 45 years, a group in which the majority of women have lost the protective effects of estrogen on the kidneys.
Regarding the relationship between age and CKD, numerous studies have identified age as a critical risk factor for CKD, with an annual increase in CKD incidence as the ages [30]. In addition, the elderly often have multiple comorbidities and require continuous medication throughout the year, contributing to an increased incidence of CKD [31]. Our study showed a stronger association between individuals aged < 60 years (OR 1.14; 95% CI 1.02–1.28; P = 0.021) and incident CKD, which may be attributed to the increased likelihood of sarcopenia with age, defined as a progressive and generalized loss of muscle mass and function commonly observed in CKD patients, especially those in end-stage hemodialysis. In the sarcopenia population, weight loss, which affects body mass index and results in lower BMI, is a common occurrence. Studies have shown that weight loss can facilitate the recovery of glomerular filtration rate, thereby reducing the incidence of CKD [32]. Since the subjects in this study did not have underlying kidney disease, body mass index influenced the TyG-BMI index, explaining the above results.
The study demonstrates a positive correlation between BMI and CKD prevalence. It is important to note the impact of obesity on CKD. Specifically, individuals with a BMI ≥ 24 kg/m2 showed a higher risk of incident CKD (OR 1.14; 95% CI 1.02–1.28; P = 0.025), as illustrated in Fig. 3.
However, our study has some limitations. Firstly, the diagnostic criterion in this study was based on eGFR rather than directly measured GFR. Direct measurement of GFR is challenging in clinical settings due to the invasive procedures, risk of drug allergy, complexity, and high cost associated with both inulin clearance and nuclear medicine radiotracer methods. Nevertheless, eGFR is an appropriate tool for clinical use. In 2012, KDIGO recommended using SCr-based equations to estimate GFR for assessing renal function. Additionally, the study's subject population was limited to China, raising questions about the generalizability of its findings to other ethnic groups. Furthermore, the study excluded individuals with pre-existing chronic kidney disease, preventing analysis of the relationship between TyG-BMI and chronic CKD progression.
Our study has the following advantages: This prospective study found high TyG-BMI is an independent risk factor for CKD, serving as a critical early warning indicator for the onset of CKD and for the screening of high-risk individuals. The TyG-BMI indicators can be easily obtained from routine peripheral whole blood test results. Its cost-effectiveness and convenience make it widely applicable in early screening, patient self-monitoring, and prevention of chronic kidney disease. We hope that future studies will include more prospective, large-scale, multicenter studies to assess the predictive value and intrinsic mechanisms of TyG-BMI for CKD.
Data availability
Data is provided within the manuscript or supplementary information files.
References
Kalantar-Zadeh K, Jafar TH, Nitsch D, Neuen BL, Perkovic V. Chronic kidney disease. Lancet. 2021;398(10302):786–802.
Levey AS. Defining AKD: the spectrum of AKI, AKD, and CKD. Nephron. 2022;146(3):302–5.
Kovesdy CP. Epidemiology of chronic kidney disease: an update 2022. Kidney Int Suppl. 2022;12(1):7–11.
Zarantonello D, Rhee CM, Kalantar-Zadeh K, Brunori G. Novel conservative management of chronic kidney disease via dialysis-free interventions. Curr Opin Nephrol Hypertens. 2021;30(1):97–107.
Jankowski J, Floege J, Fliser D, Böhm M, Marx N. Cardiovascular disease in chronic kidney disease: pathophysiological insights and therapeutic options. Circulation. 2021;143(11):1157–72.
Groopman EE, Marasa M, Cameron-Christie S, Petrovski S, Aggarwal VS, Milo-Rasouly H, Li Y, Zhang J, Nestor J, Krithivasan P. Diagnostic utility of exome sequencing for kidney disease. N Engl J Med. 2019;380(2):142–51.
Zhang X, Lerman LO. The metabolic syndrome and chronic kidney disease. Transl Res. 2017;183:14–25.
Reaven G. The metabolic syndrome or the insulin resistance syndrome? Different names, different concepts, and different goals. Endocrinol Metab Clin. 2004;33(2):283–303.
Arslanian SA, El Ghormli L, Kim JY, Tjaden AH, Barengolts E, Caprio S, Hannon TS, Mather KJ, Nadeau KJ, Utzschneider KM. OGTT glucose response curves, insulin sensitivity, and β-cell function in RISE: comparison between youth and adults at randomization and in response to interventions to preserve β-cell function. Diabetes Care. 2021;44(3):817–25.
Tahapary DL, Pratisthita LB, Fitri NA, Marcella C, Wafa S, Kurniawan F, Rizka A, Tarigan TJE, Harbuwono DS, Purnamasari D. Challenges in the diagnosis of insulin resistance: Focusing on the role of HOMA-IR and Tryglyceride/glucose index. Diabetes Metab Syndr. 2022;16(8):102581.
Son D-H, Lee HS, Lee Y-J, Lee J-H, Han J-H. Comparison of triglyceride-glucose index and HOMA-IR for predicting prevalence and incidence of metabolic syndrome. Nutr Metab Cardiovasc Dis. 2022;32(3):596–604.
Luo P, Cao Y, Li P, Li W, Song Z, Fu Z, Zhou H, Yi X, Zhu L, Zhu S. TyG index performs better than HOMA-IR in Chinese type 2 diabetes mellitus with a BMI< 35 kg/m2: a hyperglycemic clamp validated study. Medicina. 2022;58(7):876.
Malek M, Khamseh ME, Chehrehgosha H, Nobarani S, Alaei-Shahmiri F. Triglyceride glucose-waist to height ratio: a novel and effective marker for identifying hepatic steatosis in individuals with type 2 diabetes mellitus. Endocrine. 2021;74:538–45.
Hu Y, Zhao Y, Zhang J, Li C. The association between triglyceride glucose-body mass index and all-cause mortality in critically ill patients with atrial fibrillation: a retrospective study from MIMIC-IV database. Cardiovasc Diabetol. 2024;23(1):64.
Wang J, Yan S, Cui Y, Chen F, Piao M, Cui W. The diagnostic and prognostic value of the triglyceride-glucose index in Metabolic Dysfunction-Associated Fatty Liver Disease (MAFLD): a systematic review and meta-analysis. Nutrients. 2022;14(23):4969.
Ye Z, An S, Gao Y, Xie E, Zhao X, Guo Z, Li Y, Shen N, Zheng J. Association between the triglyceride glucose index and in-hospital and 1-year mortality in patients with chronic kidney disease and coronary artery disease in the intensive care unit. Cardiovasc Diabetol. 2023;22(1):110.
Rossing P, Caramori ML, Chan JC, Heerspink HJ, Hurst C, Khunti K, Liew A, Michos ED, Navaneethan SD, Olowu WA. Executive summary of the KDIGO 2022 Clinical Practice Guideline for Diabetes Management in Chronic Kidney Disease: an update based on rapidly emerging new evidence. Kidney Int. 2022;102(5):990–9.
Lv JC, Zhang LX. Prevalence and disease burden of chronic kidney disease. Renal fibrosis: mechanisms and therapies. 2019; 3–15.
Kashani K, Rosner MH, Ostermann M. Creatinine: from physiology to clinical application. Eur J Intern Med. 2020;72:9–14.
Lin B, Shao L, Luo Q, Ou-yang L, Zhou F, Du B, He Q, Wu J, Xu N, Chen J. Prevalence of chronic kidney disease and its association with metabolic diseases: a cross-sectional survey in Zhejiang province. East China BMC Nephrol. 2014;15:1–7.
Huang X, He J, Wu G, Peng Z, Yang B, Ye L. TyG-BMI and hypertension in Normoglycemia subjects in Japan: a cross-sectional study. Diab Vasc Dis Res. 2023;20(3):14791641231173616.
Huo R-R, Liao Q, Zhai L, You X-M, Zuo Y-L. Interacting and joint effects of triglyceride-glucose index (TyG) and body mass index on stroke risk and the mediating role of TyG in middle-aged and older Chinese adults: a nationwide prospective cohort study. Cardiovasc Diabetol. 2024;23(1):30.
Wang X, Liu J, Cheng Z, Zhong Y, Chen X, Song W. Triglyceride glucose-body mass index and the risk of diabetes: a general population-based cohort study. Lipids Health Dis. 2021;20:1–10.
Du Z, Xing L, Lin M, Sun Y. Estimate of prevalent ischemic stroke from triglyceride glucose-body mass index in the general population. BMC Cardiovasc Disord. 2020;20(1):483.
Jiang Y, Lai X. Association between the triglyceride glucose index, triglyceride-glucose body mass index and diabetic kidney disease in adults with newly diagnosed type 2 diabetes. Front Med. 2024;11:1328601.
Carrero JJ, Hecking M, Chesnaye NC, Jager KJ. Sex and gender disparities in the epidemiology and outcomes of chronic kidney disease. Nat Rev Nephrol. 2018;14(3):151–64.
Cobo G, Hecking M, Port FK, Exner I, Lindholm B, Stenvinkel P, Carrero JJ. Sex and gender differences in chronic kidney disease: progression to end-stage renal disease and haemodialysis. Clin Sci. 2016;130(14):1147–63.
Brar A, Markell M. Impact of gender and gender disparities in patients with kidney disease. Curr Opin Nephrol Hypertens. 2019;28(2):178–82.
Ma H-Y, Chen S, Du Y. Estrogen and estrogen receptors in kidney diseases. Ren Fail. 2021;43(1):619–42.
Pizzarelli F, Basile C, Aucella F, Dattolo PC. Chronic kidney disease in the elderly and frail patient: perspectives with opinions and comments. J Nephrol. 2023;36(6):1565–70.
Kimura H, Yoshida S, Takeuchi M, Kawakami K. Impact of potentially inappropriate medications on kidney function in chronic kidney disease: retrospective cohort study. Nephron. 2023;147(3–4):177–84.
Nakanishi S, Iwamoto M, Shinohara H, Iwamoto H, Kaneto H. Significance of body mass index for diagnosing sarcopenia is equivalent to slow gait speed in Japanese individuals with type 2 diabetes: cross-sectional study using outpatient clinical data. J Diabetes Investig. 2021;12(3):417–24.
Acknowledgements
Thanks to the participants and staff in this study for their valuable contributions.
Funding
This work was supported by the National Natural Science Foundation of China, No. 82101662.
Author information
Authors and Affiliations
Contributions
ATH and FC drafted the first version of the manuscript, which was critically revised by WZH. Data collection and statistical analyses were performed by FC, GMY, and CSY. The final version of the manuscript was reviewed and approved by all authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
All research activities were conducted in accordance with the guidelines of the Medical Ethics Committee of 2013 and the Declaration of Helsinki, and received approval from the Medical Ethics Committee of Tongji Medical College, Huazhong University of Science and Technology. Written informed consent was obtained from all participants before their inclusion in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Fan, C., Guo, M., Chang, S. et al. Elevated TyG-BMI index predicts incidence of chronic kidney disease. Clin Exp Med 24, 203 (2024). https://doi.org/10.1007/s10238-024-01472-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10238-024-01472-3