Abstract
As one of the factors regulating tumour angiogenesis, angiopoietin-4 (ANGPT4), which plays an important role in promoting tumour proliferation, survival, expansion and angiogenesis, is highly expressed in some tumours, such as lung adenocarcinoma, glioblastoma and ovarian cancer. This may be related to the fact that ANGPT4 affects the blood vessels and lymphatic system of the tumour. Specifically, ANGPT4 could play an effective role in promoting cancer by affecting the tyrosine kinase receptor TIE2, ERK1/2 and PI3K/AKT signalling pathways. Therefore, ANGPT4 may be an important biomarker for the occurrence and development of cancer and poor prognosis. In addition, the inhibition of ANGPT4 may be a useful cancer treatment. This paper reviews the latest preclinical research on ANGPT4, emphasizes its role in tumourigenesis and broadens our understanding of the carcinogenic function of ANGPT4 and the development of ANGPT4 inhibitors. This is the latest version of the revised version of the previous article.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Angiopoietin (ANGPT) family proteins are a class of glycoproteins closely related to angiogenesis and include the murine angiopoietin protein ANGPT3 and three humanized angiopoietin proteins: ANGPT1, ANGPT2 and ANGPT4 [1]. The angiopoietin family typically participates in tumourigenesis and development by activating the tyrosine kinase receptor TIE2, which can affect the survival, proliferation and migration of many kinds of cells in the tumour microenvironment (TME) [2]. These proteins act mainly on the angiogenesis system and play a significant role in controlling physiological and pathological angiogenesis regulation and stability [1, 3, 4], participating in the appearance and progression of cancer, autoimmune diseases and other diseases [5,6,7].
Some studies have shown that the widespread expression of ANGPT1 in a physiological state plays a significant role in angiogenic remodelling and vessel maturation and stabilization [8,9,10], whereas its overexpression in different types of tumour-associated angiogenesis promotes and inhibits angiogenesis [11]. ANGPT2 is rarely expressed in the physiological state but is overexpressed in the vasculature of multiple tumours and actively promotes the proliferation, invasion and angiogenesis of these tumours, including glioma, mammary carcinoma, lung carcinoma, gastric cancer, colon cancer, prostate cancer, oesophageal squamous cell carcinoma, hepatocellular carcinoma and melanoma [11, 12]. ANGPT3 is overexpressed in glioblastoma and lung tumour models established in animal experiments and promotes their occurrence and development [1, 13, 14]; however, its overexpression in a breast carcinoma pulmonary metastasis model and a spontaneous pulmonary metastasis model using a subline of Lewis lung carcinoma cells inhibits pulmonary metastasis [15]. Importantly, the nomenclature ANGPT3 is no longer in use, as the mouse form is also referred to as ANGPT4 according to the gene nomenclature guidelines of the Hugo Gene Nomenclature Committee (HGNC, www.genenames.org) and the Mouse Genome Informatics (MGI, www.Informatics.jax.org). Interestingly, several recent studies have reported a role for ANGPT4 in the eye and heart organs of mice. ANGPT4 in the retina of mice has been reported to play an important role in the development or maintenance of retinal veins and Schlemm’s canal in mice [7, 16], and it has also been reported to promote cardiac regeneration and repair after myocardial infarction in mice [17]. ANGPT4 protein is synthesized within cells and then secreted outside the cell, and ANGPT4 expression can regulate the microenvironment and promote tumour cell migration, proliferation and angiogenesis in various kinds of cancers, such as lung adenocarcinoma [4, 18], breast cancer [6], Kaposi’s sarcoma [19], ovarian cancer [20], glioblastoma [21], lymphoma [22, 23], pancreatic cancer [24], renal clear cell carcinoma [25], prostate cancer [26], colorectal adenocarcinoma [27], gastric carcinoma [28], gastrointestinal stromal tumour, leiomyoma and schwannoma [29].
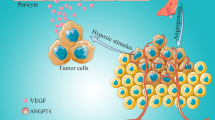
The formation of the vascular system is based on the response of ECs to angiogenic stimuli, such as vascular endothelial growth factors, fibroblast growth factors and angiopoietins, and is produced through the processes of vascular budding, cell migration and proliferation, vascular maturation and vascular interconnection. Because angiogenesis is relatively static after the vascular system matures, the balance between activating and inhibiting signalling pathways is strictly regulated. Thus, only under pathological processes, including inflammation, atherosclerosis, restenosis, many types of vasculopathy and cancer will this balance be disrupted and shifted towards angiogenesis. In addition, although the angiogenesis mechanism and process are complex, angiogenesis shares many cellular and molecular processes in both the pathological and physiological states [1]. ANGPT-TIE2 is an indispensable part of these molecular pathways. However, the roles of these three ANGPTs in tumour angiogenesis may be complex and involve crosstalk (Fig. 1). In short, when stimulated by hypoxia or HIF-1α, the expression of ANGPT4 in vascular endothelial cells [30] and tumour cells [25] is increased, which can promote the proliferation, survival, migration and branch formation of vascular endothelial cells by increasing the phosphorylation of TIMEs. In addition, ANGPT4 has been shown to reduce the coverage of vascular pericytes [21]. Moreover, ANGPT1 can promote the survival of endothelial cells and vascular stability by promoting the aggregation and activation of TIE2 [31, 32]. Moreover, ANGPT2 stimulated by hypoxia or VEGF also begins to combine with TIE2 at the site of angiogenesis to activate endothelial cells, causing some endothelial cells to detach from pericytes and begin to sprout tip cells [33]. In addition, other studies have shown that the expression of ANGPT4 in tumours may regulate the occurrence and development of tumours by affecting the PI3K-Akt signalling pathway [23], the Hedgehog signalling pathway [26], the ERK signalling pathway [34], the MAPK signalling pathway and the Ras signalling pathway [17]. These conclusions indicate that ANGPT4 is not only an interesting diagnostic marker but also a meaningful therapeutic target for many cancers.
Mechanism by which ANGPT4 promotes the formation of the tumour vascular system. The expression of ANGPT4 in endothelial cells and tumour cells increases after stimulation by vascular demand, such as by tumour hypoxia. ANGPT4 binds to TIE2 on endothelial cells related to angiogenesis and promotes the stability, survival, migration and tubule formation of vascular endothelial cells. Moreover, ANGPT4 can reduce the coverage of extravascular pericytes and facilitate the sprouting of vascular endothelial cells
To further stimulate interest in the related research on ANGPT4, we discuss all the known carcinogenic mechanisms related to ANGPT4, especially in tumour angiogenesis. This is the latest version of the revised version of the previous article [35].
Brief description of ANGPT4
ANGPT4, a regulator of angiogenesis, plays an important regulatory role in angiogenesis and expansion [21]. Moreover, the associations between ANGPT4 expression and cancer progression and prognosis indicate that information related to ANGPT4 should be considered and further explored by researchers [34].
Protein structure of ANGPT4
According to the NIH database, the human ANGPT4 gene is located on chromosome 20p13. However, the human ANGPT1 gene is located at 8q23, and the ANGPT2 gene is located at 8p23. The ANGPT4 protein is encoded by a single 4-kb messenger RNA (mRNA) [3], which encodes 503 amino acid long chains with a predicted molecular mass of 56.8 kDa. Nonetheless, an S-labelled reaction product had a protein weight of 72 kDa [36]. The differences in the molecular weight of the ANGPT4 protein may be due to its configurational characteristics. ANGPT4, a member of the ANGPT family, is consistent with other members in the main structure [1, 3]. The main structure of ANGPT is divided into four parts: an N-terminal superclustering domain, a central coiled-coil domain responsible for ligand homo-oligomerization, a linker region and a C-terminal fibrinogen-like domain required for binding to the TIE2 receptor [1, 3]. In addition, ANGPT4, similar to other ANGPTs, maintains a pattern of three closely spaced cysteines and retains a signature cysteine-based motif in its fibrinogen-like domain that appears to mark angiopoietins rather than other fibrinogen-like sequences [3]. Notably, the mouse ANGPT4 gene, located on mouse chromosome 2, shares 65% of the amino acids of the fibrinogen-like structural domain with human ANGPT4. This difference in gene expression is greater than the difference between mouse and human ANGPT1 and ANGPT2, which share 99% and 87% of the amino acids in the fibrinogen-like structural domain [3]. Additionally, ANGPT4, under non-reducing conditions, mostly forms a characteristic disulphide-linked dimer and rarely forms a variable higher-order multimer [36].
ANGPT4-TIE2 signalling pathway
The more recognized classical regulatory pathway of the ANGPT4 protein is the ANGPT-TIE2 receptor pathway. ANGPT4 is usually considered an activator of the TIE2 pathway (Fig. 2), which regulates angiogenesis, and promotes cell survival by acting on this pathway [3]. However, it has also been shown that monomeric/dimeric ANGPT4 does not directly activate the TIE2 receptor [37], which may be related to the characteristic disulphide bond linkage of the ANGPT4 dimer structure, as TIE2 requires higher-order multimers for activation [36]. The findings of Brunckhorst et al. may be consistent with this view, as they reported that ANGPT4, which promotes glioblastoma angiogenesis and tumour cell proliferation, was present in an aggregated state in their experiments, and ERK1/2, a downstream signalling molecule normally mediated by TIE2, was significantly activated, thereby promoting glioblastoma cell survival and activity [21]. This may reflect the different aggregation states of ANGPT4 expressed by different tumour cells and/or modified by different tumour microenvironments, with monomeric/dimeric ANGPT4 inhibiting tumour angiogenesis and aggregated ANGPT4 promoting tumour angiogenesis [21, 37]. However, the mechanism by which the ANGPT4/TIE2 pathway promotes tumour angiogenesis is complex and may involve a joint role for ANGPT4, ANGPT1 and ANGPT2, as mentioned in the introduction. In addition, ANGPT4 was shown to activate the ANGPT-TIE2 functional axis not only by blocking the ANGPT2 and TIE2 pathways but also by enhancing tumour cell invasion of host organs by establishing a protumour microenvironment in a paracrine manner in endothelial cells and in an autocrine manner in tumour cells and by promoting ovarian cancer-associated fibroblastic fine (OCAF) aggregation [20]. A previous study suggested that ANGPT4 is likely to function biologically similarly to ANGPT1 (another TIE2 agonist) by acting on TIE2, thereby allowing PI3K/Akt signalling to be activated, which, in turn, promotes tumour cell survival and antiapoptotic signalling by upregulating Survivin and endothelial nitric oxide synthase (eNOS) as well as inhibiting Caspase-9 and Bad [38].
Potential carcinogenic mechanism of ANGPT4. ANGPT4 expression and activation are mediated by HIF-1, Hedgehog (HG) and YB-1. Furthermore, NVP-BKM120 inhibits ANGPT4 expression in cancer. Oligomeric ANGPT4 enables the TIE2 axis to strongly express the TIE2 downstream signalling molecules PIK3 and ERK1/2 to promote tumour survival and antiapoptotic signalling while promoting EC quiescence, survival and vascular stability
Notably, ANGPT4 may be involved in more than just the ANGPT4-TIE2 signalling pathway. ANGPT4 was shown to play a key role in myocardial regeneration and repair through the MAPK and retinoic acid pathways in a recent study, which revealed that ANGPT4 can upregulate the oncogenes KRAS and NRAS [17]. Moreover, considering that both ANGPT1 [39, 40] and ANGPT2 [41] in the ANGPT family can exert their functions through binding with the integrin family, we speculated that the functions of ANGPT4 and the related pathways it participates in are rich and diverse; thus, ANGPT4 may be more interesting than TIE2 as a target in tumour therapy.
Regulation of ANGPT4
The expression of ANGPT4 in tumours is regulated mainly by hypoxia and HIF-1α, and some studies have shown that the mechanisms regulating ANGPT4 expression may be rich and diverse [24, 25, 30]. A previous study on prostate tumour signalling pathways suggested that significant expression of ANGPT4 may be mediated by the involvement of the paracrine or autocrine ligand Sonic Hedgehog through the Hedgehog signalling pathway [26]. A recent study suggested that ANGPT4 mRNA and protein expression may be activated by the binding of Y-box binding protein 1 (YB-1) to its promoter, which can promote capillary formation in non-small cell carcinoma [18]. In addition, a DNA methylation study of genes associated with birthweight indicated that ANGPT4 expression and its biological function in disease may also be regulated by the methylation of related genes [42]. Taken together, the rich regulatory mechanisms and roles of the ANGPT4 gene upstream and downstream lay the foundation for its use as a promising therapeutic target in oncology research.
ANGPT4 expression promotes tumour angiogenesis and tumour proliferation
In previous studies, ANGPT4 was reported to be overexpressed in various human cancers [6, 18,19,20,21,22,23,24,25,26,27,28,29], and a relationship between ANGPT4 expression and clinical characteristics was also observed. Cong et al. reported that ANGPT4 is overexpressed in patients with non-small cell lung cancer and that the activation of ANGPT4 may promote tumour microvessel density, suggesting that ANGPT4 may be an attractive antiangiogenic target for antitumour therapy [18]. Yang et al. reported that genes positively correlated with ANGPT4 were significantly enriched in breast cancer angiogenesis and that the DNA copy number of the ANGPT4 gene was increased. They also reported that ANGPT4 was significantly correlated with the breast cancer microenvironment score and the development of tumour-associated fibroblasts and endothelial cells and was positively correlated with the overall survival rate of breast cancer patients [6]. Brown et al. found through skin tissue biopsy that there is ectopic expression of ANGPT4 in some Kaposi’s sarcoma tissues, which may indicate that inhibiting ANGPT4 and Tie receptors can aid in its treatment [19]. Brunckhorst et al. confirmed that the overexpression of ANGPT4 promoted tumour angiogenesis and glioblastoma multiforme progression and suggested that ANGPT4 may play an important role in the conversion of proangiogenic signalling pathways in glioblastoma multiforme, particularly in the resistance to antiangiogenic treatments [21]. In addition, Brunckhorst et al. reported that ANGPT4 is expressed in both tumour cells and the stroma of ovarian cancer patients, but it primarily sends a strong signal in the tumour stroma. Moreover, they reported that ANGPT4 may play a role in establishing and promoting the microenvironment outside of tumour cells, such as promoting tumour angiogenesis and the accumulation of tumour-associated fibroblasts in ovarian tumours, thereby promoting cancer migration and invasion [20]. In conclusion, their study revealed that ANGPT4 promoted the subcutaneous and intracranial growth of glioblastoma multiforme and ovarian cancer, whereas downregulating the expression of ANGPT4 inhibited the proliferation of these tumours and significantly improved the survival rate of patients [20, 21]. Ren et al. reported that ANGPT4 is highly expressed in patients with NK/T-cell lymphoma-associated haemophagocytic syndrome, suggesting that ANGPT4 is a potential cause of severe inflammatory storms in such patients [22]. Gupta et al. demonstrated that ANGPT4 expression in pancreatic cancer often indicates that pancreatic cancer patients are suffering from hypoxic stimulation, which can predict poor clinical outcomes [24]. Yamakawa et al. reported that ANGPT4 expression was increased in renal clear cell carcinoma and that there was a significant difference in ANGPT4 expression between normal renal proximal tubule epithelial cells under normoxic conditions; however, ANGPT2 did not reflect this difference. Moreover, under hypoxic stress conditions, ANGPT4 levels are significantly increased, and the related cell-conditioned medium promotes angiogenesis [25]. Shaw et al. proposed that the ANGPT4 gene, which promotes tumour growth, is one of the nine significantly expressed genes in the prostate cancer stroma [26]. Nakayama et al. reported that the expression of ANGPT4 was significantly related to several types of tissue differentiation and several clinicopathological types of gastric cancer, suggesting that the ANGPT4 protein may be one of the precursor factors in gastric cancer [28]. In addition, Nakayama et al. reported that ANGPT4 is almost not expressed in normal colon epithelial tissue, but the expression of ANGPT4 in colorectal adenocarcinoma is related to the depth of tumour invasion, Duke’s grade, venous invasion and lymphatic invasion [27]. Moreover, researchers have reported that ANGPT4, which may play an important role, is expressed to varying degrees in gastrointestinal stromal tumours, leiomyomas and schwannomas [29]. Kesler et al. reported that the overexpression of ANGPT4 promoted angiogenesis and haematogenous metastasis in fibrosarcoma [34]. Although current research on the mechanism of action of ANGPT4 is limited, the expression level of ANGPT4 is closely related to the occurrence and development of various cancers and can be used as an important indicator for the diagnosis and prognosis of cancer patients. In summary, ANGPT4 is likely to promote the proliferation, migration, invasion and metastasis of various cancer cells, suggesting that ANGPT4 is a potential target for antitumour angiogenesis and the inhibition of tumour growth and invasion.
Effects of ANGPT4 on lymphatic vessel formation, dilation, permeability and metastasis
Lymphatic vessels and pathways play significant roles in the tumour microenvironment and tumour metastasis [43, 44]. Previous studies have shown that the ANGPT/Tie system also plays a significant role in the remodelling and maturation of lymphatic vessels. For example, ANGPT1 induces lymphatic vessel enlargement, germination and proliferation by activating TIE2 in a vascular endothelial growth factor receptor 3 (VEGFR-3)-dependent manner [45]; the ANGPT2/TIE2 functional axis appears to be involved in lymphatic vessel remodelling and stabilization; and a genetic study revealed that ANGPT2-deficient mice present more severe defects in the lymphatic vessel system than vascular defects do [46]. In view of the manifestations of ANGPT1 and ANGPT2 in lymphatic vessels, exploring the alterations of ANGPT4 in lymphangiogenesis, dilation and permeability could help in the study of antitumour lymphatic tract metastasis therapy. ANGPT4 promotes the proliferation and activity of tumour lymphatic and vascular endothelial cells and expands the diameter of lymphatic vessels, but there is no significant difference in the increase in the monolayer permeability of lymphatic vessels. The dilation effect of ANGPT4 on lymphatic vessels is secondary to the changes in vasodilation and permeability. These findings indicate that the mechanism by which ANGPT4 affects lymphatic vessels is complex and that blocking ANGPT4 may damage the draining lymphatic system and reduce vascular permeability [34]. Therefore, blocking ANGPT4 in lymphatic vessels and reducing vascular permeability may help limit the metastasis of cancer and improve the release of tumour drugs by decreasing interstitial fluid pressure [37]. Kim et al. expressed ANGPT4 via an adenoviral vector system and reported that the overexpression of ANGPT4 increased the number of lymphatic filamentous pseudopods in the trachea and the lymphatic density in healing wounds [47]. In summary, although few studies have investigated the role and mechanism of ANGPT4 in lymphatic vessels and development, the available studies suggest that the inhibition of ANGPT4 expression helps to inhibit tumour lymphatic vascular activity and survival and even affects the tumour vasculature by inhibiting the lymphatic vasculature. Additionally, further studies on the mechanism of ANGPT4 action on tumour lymphatics could help to discover whether ANGPT4 plays an immunological role and mechanism in tumours.
ANGPT4 in preclinical studies
Because the role of ANGPT in tumourigenesis suggests the importance of ANGPT as a tumour therapeutic target, the development of corresponding ANGPT inhibitors is promising. Inhibitors targeting the ANGPT family include the inhibitors MEDI-3617 and nesvacumab, which specifically inhibit ANGPT2, as well as trebananib and AMG780, which target ANGPT1 and ANGPT2 [48]. In addition, RO5520985 and BI836880 can inhibit ANGPT2 and VEGF at the same time [48, 49]. Therefore, the high expression and biological role of ANGPT4 in a variety of tumours suggest that it not only has diagnostic value but also may play a role in identifying tumour treatment targets and improving patient prognosis. By using the powerful Pan-PI3K inhibitor NVP-BKM12018, researchers demonstrated that ANGPT4, a component of the PI3K/AKT pathway in follicular lymphoma, was downregulated [23]. ANGPT4 is upregulated by risk factors, according to a recent study of the main risk factors for lung cancer; however, garlic chemical mixture (DADS) might suppress ANGPT4 overexpression [50]. Intriguingly, a previous study revealed that low-level laser therapy can enhance bone repair by upregulating the expression of angiogenic factors, such as ANGPT4; therefore, it is important to consider whether we should examine the impact of this type of laser on tumour angiogenesis [51]. Preclinical investigations of molecules closely related to ANGPT4 should be focused on, in addition to preclinical studies of inhibitors that affect ANGPT4, since this will help to advance our understanding of ANGPT4. An earlier study on lung adenocarcinoma demonstrated that LINC00665, which may be a key factor in the resistance of cancer cells to gefitinib, cisplatin and gemcitabine, can bind to and stabilize YB-1, which, in turn, can activate the transcription of several genes linked to cancer progression and multidrug resistance and can regulate and increase the expression of ANGPT4 [18]. However, while ANGPT4 is targeted for cancer therapy, the aggregated forms of ANGPT4 may also need to be investigated. As mentioned above, Olsen et al. and Xu et al. reported that ANGPT4 may inhibit angiogenesis and tumour progression [15, 37], although Brunckhorst et al. and other researchers reported that this inhibitory effect may be because ANGPT4 exists in monomers or dimers under reducing conditions and cannot form greater aggregates [21, 52]. Overall, it is worthwhile to explore ANGPT4 inhibitors and their related manifestations before and after radiotherapy for tumour prevention and prognosis.
Conclusion and prospects
This article generalizes the expression, configurations and biological effects of ANGPT4 in multiple cancers, which are sophisticated and interesting and have remained indistinct to date. Less is known about the roles of ANGPT4 in other characteristics of tumours, such as transcriptional regulation, epigenetic modification, immune escape, protein chemical modification and energy metabolism disorders. Among them, the regulatory role of ANGPT4 in the immune response of cancer should also be taken seriously. Recently, a study of NK/T-cell lymphoma-associated phagocytic syndrome (NK/T-LAHS) revealed that ANGPT4 plays a significant role in the activation of inflammatory cells in the inflammatory response, possibly by interacting with megakaryocytes to alter alpha granule content and by promoting specific platelet production, thereby promoting inflammation through the secretion of alpha granules containing upregulated platelet factor 4 (PF4) and downregulated platelet-derived growth factors (PDGFs) [22]. The molecular mechanisms of the different effects of ANGPT4 on tumour formation and progression have not been completely determined. Therefore, exploring ANGPT4 and its signalling pathway will help construct a complete cancer signalling network.
Moreover, the mechanism of ANGPT4 overexpression and reactivation in cancer remains uncertain. The upstream factors regulating the activation of ANGPT4 are not consistent across different types of cancer; for example, HIF-1 is correlated with ectopic and reactivated ANGPT4 in renal clear cell carcinoma [25], Hedgehog in prostate tumours [26] and YB-1 in lung non-small cell carcinoma [18]. Hence, understanding the role of ANGPT4 in other genetic or epigenetic modifications that trigger the development and progression of cancer is important for cancer prevention and control. Furthermore, ANGPT4 is one of the targets of antitumour angiogenesis and lymphangiogenesis, and ANGPT4 may also be a therapeutic target for VEGF-targeted drug resistance [21]. ANGPT4 is known to maintain angiogenesis and lymphangiogenesis. Biological behaviours, such as proliferation, metastasis, invasion and recurrence, are affected by neovascularization and disorders of tumour blood vessels and lymphatic vessels. Clarifying the role of ANGPT4 in this context will contribute to the treatment of cancer and the determination of effective cancer biomarkers.
Reference
Fagiani E, Christofori G. Angiopoietins in angiogenesis. Cancer Lett. 2013;328:18–26.
He FF, Zhang D, Chen Q, et al. Angiopoietin-Tie signaling in kidney diseases: an updated review. FEBS Lett. 2019;593:2706–15.
Valenzuela DM, Griffiths JA, Rojas J, et al. Angiopoietins 3 and 4: diverging gene counterparts in mice and humans. Proc Natl Acad Sci U S A. 1999;96:1904–9.
Tsakogiannis D, Nikolakopoulou A, Zagouri F, et al. Update overview of the role of angiopoietins in lung cancer. Medicina (Kaunas). 2021;57:1191.
Wu Q, Xu WD, Huang AF. Role of angiopoietin-2 in inflammatory autoimmune diseases: A comprehensive review. Int Immunopharmacol. 2020;80:106223.
Yang H, Zhang M, Mao XY, et al. Distinct clinical impact and biological function of angiopoietin and angiopoietin-like proteins in human breast cancer. Cells. 2021;10:2590.
Elamaa H, Kihlström M, Kapiainen E, et al. Angiopoietin-4-dependent venous maturation and fluid drainage in the peripheral retina. Elife. 2018. https://doi.org/10.7554/eLife.37776.
Davis S, Aldrich TH, Jones PF, et al. Isolation of angiopoietin-1, a ligand for the TIE2 receptor, by secretion-trap expression cloning. Cell. 1996;87:1161–9.
Sato TN, Tozawa Y, Deutsch U, et al. Distinct roles of the receptor tyrosine kinases Tie-1 and Tie-2 in blood vessel formation. Nature. 1995;376:70–4.
Suri C, Jones PF, Patan S, et al. Requisite role of angiopoietin-1, a ligand for the TIE2 receptor, during embryonic angiogenesis. Cell. 1996;87:1171–80.
Thomas M, Augustin HG. The role of the Angiopoietins in vascular morphogenesis. Angiogenesis. 2009;12:125–37.
Hu B, Cheng SY. Angiopoietin-2: development of inhibitors for cancer therapy. Curr Oncol Rep. 2009;11:111–6.
Beaudet MJ, Rueda N, Kobinger GP, et al. Construction of a ganciclovir-sensitive lentiviral vector to assess the influence of angiopoietin-3 and soluble Tie2 on glioma growth. J Neurooncol. 2010;99:1–11.
Zhang S, Chen Z, Yang J, et al. Angiopoietin-3 overexpression in tobacco smoke-induced mouse lung tumors and its relation to vitamin E intervention. Mol Med Rep. 2008;1:729–33.
Xu Y, Liu YJ, Yu Q. Angiopoietin-3 inhibits pulmonary metastasis by inhibiting tumor angiogenesis. Cancer Res. 2004;64:6119–26.
Kapiainen E, Elamaa H, Miinalainen I, et al. Cooperation of Angiopoietin-2 and Angiopoietin-4 in Schlemm’s Canal Maintenance. Invest Ophthalmol Vis Sci. 2022;63:1.
Wu Z, Shi Y, Cui Y, et al. Single-cell analysis reveals an Angpt4-initiated EPDC-EC-CM cellular coordination cascade during heart regeneration, Protein. Cell. 2023;14:350–68.
Cong Z, Diao Y, Li X, et al. Long non-coding RNA linc00665 interacts with YB-1 and promotes angiogenesis in lung adenocarcinoma. Biochem Biophys Res Commun. 2020;527:545–52.
Brown LF, Dezube BJ, Tognazzi K, et al. Expression of Tie1, Tie2, and angiopoietins 1, 2, and 4 in Kaposi’s sarcoma and cutaneous angiosarcoma. Am J Pathol. 2000;156:2179–83.
Brunckhorst MK, Xu Y, Lu R, et al. Angiopoietins promote ovarian cancer progression by establishing a procancer microenvironment. Am J Pathol. 2014;184:2285–96.
Brunckhorst MK, Wang H, Lu R, et al. Angiopoietin-4 promotes glioblastoma progression by enhancing tumor cell viability and angiogenesis. Cancer Res. 2010;70:7283–93.
Ren Q, Chan KW, Huang H, et al. Platelet-derived alpha-granules are associated with inflammation in patients with NK/T-cell lymphoma-associated hemophagocytic syndrome. Cytokine. 2020;126:154878.
Matas-Céspedes A, Rodriguez V, Kalko SG, et al. Disruption of follicular dendritic cells-follicular lymphoma cross-talk by the pan-PI3K inhibitor BKM120 (Buparlisib). Clin Cancer Res. 2014;20:3458–71.
Gupta N, Park JE, Tse W, et al. ERO1α promotes hypoxic tumor progression and is associated with poor prognosis in pancreatic cancer. Oncotarget. 2019;10:5970–82.
Yamakawa M, Liu LX, Belanger AJ, et al. Expression of angiopoietins in renal epithelial and clear cell carcinoma cells: regulation by hypoxia and participation in angiogenesis. Am J Physiol Renal Physiol. 2004;287:F649-657.
Shaw A, Gipp J, Bushman W. The Sonic Hedgehog pathway stimulates prostate tumor growth by paracrine signaling and recapitulates embryonic gene expression in tumor myofibroblasts. Oncogene. 2009;28:4480–90.
Nakayama T, Hatachi G, Wen CY, et al. Expression and significance of Tie-1 and Tie-2 receptors, and angiopoietins-1, 2 and 4 in colorectal adenocarcinoma: Immunohistochemical analysis and correlation with clinicopathological factors. World J Gastroenterol. 2005;11:964–9.
Nakayama T, Yoshizaki A, Kawahara N, et al. Expression of Tie-1 and 2 receptors, and angiopoietin-1, 2 and 4 in gastric carcinoma; immunohistochemical analyses and correlation with clinicopathological factors. Histopathology. 2004;44:232–9.
Nakayama T, Inaba M, Naito S, et al. Expression of angiopoietin-1, 2 and 4 and Tie-1 and 2 in gastrointestinal stromal tumor, leiomyoma and schwannoma. World J Gastroenterol. 2007;13:4473–9.
Yamakawa M, Liu LX, Date T, et al. Hypoxia-inducible factor-1 mediates activation of cultured vascular endothelial cells by inducing multiple angiogenic factors. Circ Res. 2003;93:664–73.
Fukuhara S, Sako K, Minami T, et al. Differential function of Tie2 at cell-cell contacts and cell-substratum contacts regulated by angiopoietin-1. Nat Cell Biol. 2008;10:513–26.
Saharinen P, Eklund L, Miettinen J, et al. Angiopoietins assemble distinct Tie2 signalling complexes in endothelial cell-cell and cell-matrix contacts. Nat Cell Biol. 2008;10:527–37.
Hammes HP, Lin J, Wagner P, et al. Angiopoietin-2 causes pericyte dropout in the normal retina: evidence for involvement in diabetic retinopathy. Diabetes. 2004;53:1104–10.
Kesler CT, Pereira ER, Cui CH, et al. Angiopoietin-4 increases permeability of blood vessels and promotes lymphatic dilation. Faseb j. 2015;29:3668–77.
Zhou WC, Zhang QF, Chen JL, et al. Retraction Note: angiopoietin4 (ANGPT4) expression and potential mechanisms in carcinogenesis: current achievements and perspectives. Clin Exp Med. 2024;24:71.
Lee HJ, Cho CH, Hwang SJ, et al. Biological characterization of angiopoietin-3 and angiopoietin-4. Faseb j. 2004;18:1200–8.
Olsen MW, Ley CD, Junker N, et al. Angiopoietin-4 inhibits angiogenesis and reduces interstitial fluid pressure. Neoplasia. 2006;8:364–72.
Duran CL, Borriello L, Karagiannis GS, et al. Targeting Tie2 in the tumor microenvironment: from angiogenesis to dissemination. Cancers (Basel). 2021;13:5730.
Chen X, Fu W, Tung CE, et al. Angiopoietin-1 induces neurite outgrowth of PC12 cells in a Tie2-independent, beta1-integrin-dependent manner. Neurosci Res. 2009;64:348–54.
Dallabrida SM, Ismail NS, Pravda EA, et al. Integrin binding angiopoietin-1 monomers reduce cardiac hypertrophy. Faseb j. 2008;22:3010–23.
Imanishi Y, Hu B, Jarzynka MJ, et al. Angiopoietin-2 stimulates breast cancer metastasis through the alpha(5)beta(1) integrin-mediated pathway. Cancer Res. 2007;67:4254–63.
Turan N, Ghalwash MF, Katari S, et al. DNA methylation differences at growth related genes correlate with birth weight: a molecular signature linked to developmental origins of adult disease? BMC Med Genomics. 2012;5:10.
Petrova TV, Koh GY. Organ-specific lymphatic vasculature: From development to pathophysiology. J Exp Med. 2018;215:35–49.
Tammela T, Alitalo K. Lymphangiogenesis: Molecular mechanisms and future promise. Cell. 2010;140:460–76.
Tammela T, Saaristo A, Lohela M, et al. Angiopoietin-1 promotes lymphatic sprouting and hyperplasia. Blood. 2005;105:4642–8.
Gale NW, Thurston G, Hackett SF, et al. Angiopoietin-2 is required for postnatal angiogenesis and lymphatic patterning, and only the latter role is rescued by Angiopoietin-1. Dev Cell. 2002;3:411–23.
Kim KE, Cho CH, Kim HZ, et al. In vivo actions of angiopoietins on quiescent and remodeling blood and lymphatic vessels in mouse airways and skin. Arterioscler Thromb Vasc Biol. 2007;27:564–70.
Sheridan C. Amgen’s angiopoietin blocker fails in ovarian cancer. Nat Biotechnol. 2015;33:5–6.
Hofmann I, Baum A, Hofmann MH, et al. Pharmacodynamic and Antitumor Activity of BI 836880, a Dual Vascular Endothelial Growth Factor and Angiopoietin 2 Inhibitor, Alone and Combined with Programmed Cell Death Protein-1 Inhibition. J Pharmacol Exp Ther. 2023;384:331–42.
Hudlikar RR, Chou PJ, Kuo HD, et al. Long term exposure of cigarette smoke condensate (CSC) mediates transcriptomic changes in normal human lung epithelial Beas-2b cells and protection by garlic compounds. Food Chem Toxicol. 2023;174:113656.
Tim CR, Bossini PS, Kido HW, et al. Effects of low level laser therapy on inflammatory and angiogenic gene expression during the process of bone healing: A microarray analysis. J Photochem Photobiol B. 2016;154:8–15.
Xu Y, Liu YJ, Yu Q. Angiopoietin-3 is tethered on the cell surface via heparan sulfate proteoglycans. J Biol Chem. 2004;279:41179–88.
Funding
The present study was supported by the Natural Science Foundation of HuNan Province (2021JJ50070 and 2023JJ41066) and the Key Project of Scientific Research Project of HuNan Provincial Education Department (22A0317).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. WC Zhou, JL Chen and QF Zhang designed the project. WC Zhou, JL Chen and JP Gan wrote the paper. YK Li drawing the graph. YK Li and J Zou revised the manuscript and designed the experiment.
Corresponding authors
Ethics declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Consent to participate
Not applicable.
Consent to publish
Not applicable.
Ethics approval
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Zhou, W., Zhang, Q., Chen, J. et al. Angiopoietin-4 expression and potential mechanisms in carcinogenesis: Current achievements and perspectives. Clin Exp Med 24, 224 (2024). https://doi.org/10.1007/s10238-024-01449-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10238-024-01449-2