Abstract
Although immune checkpoint inhibitors (ICIs) have gained approval for metastatic renal cell carcinoma (mRCC), the response rate is still limited. Therefore, it is urgent to explore novel markers of responses to ICIs that can help assess clinical benefits. Recently, it has been noted that peripheral blood eosinophil counts are an independent factor correlated with clinical outcome of ICIs in some types of cancer. We investigated peripheral blood absolute eosinophil counts (AECs) at baseline and 4 weeks after the initiation of nivolumab for mRCC patients between February 2016 and May 2022. In addition, we examined clinicopathological features including irAEs and analyzed the correlation between AECs and clinical efficacy of nivolumab. The median progression-free survival (PFS) and overall survival (OS) for all patients were 5.7 and 25.5 months, respectively. The median AECs in patients with irAEs were significantly higher at baseline and 4 weeks after the treatment compared to those without irAEs (p < 0.001 and p = 0.001). With the cutoff value of AECs of 329 cells/µL at 4 weeks after the treatment for prediction of irAEs, high-AECs groups had significantly higher number of responders compared with that in low-AECs group (p < 0.001). Accordingly, the PFS and OS were significantly better in patients with high-AECs group than those in low-AECs group (p = 0.03 and p = 0.009). High-AECs at 4 weeks after the treatment serve as the prominent surrogate marker associated with the incidence of irAEs and better clinical outcome in mRCC patients receiving nivolumab.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Renal cell carcinoma (RCC) accounts for about 2–4% of all types of cancer worldwide [1]. The 5-year specific survival in patients with early stage is reported to be 90%, but once metastasized, the 5-year survival plummets to 14%, although 17% of RCC patients present with the evidence of distant metastasis at initial diagnosis [2, 3]. In addition, 20% of RCC patients who undergo surgical resection of localized RCC eventually develop distant metastases, necessitating subsequent therapeutic interventions including immune checkpoint inhibitors (ICIs)-based treatments [4].
ICIs have been widely used in the treatment of many types of cancer and have remarkably improved the prognosis of cancer patients. However, the majority of patients may not benefit from the therapy with ICIs and sometimes experience severe immune-related adverse events (irAE). To date, numerous biomarkers, including PD-L1 expression, tumor mutation burden, and tumor-infiltrating lymphocytes in cancer tissues, have been suggested to play a crucial role in influencing the therapeutic response to ICIs [5]. However, these markers mainly focus on the tumor state at the time of diagnosis and sometimes produce contradictory outcomes. Therefore, there is an urgent need for noninvasive biomarkers that can predict the efficacy of ICIs, aiming to prevent unnecessary treatments.
Various factors have been investigated to predict response, and an association between neutrophil lymphocyte ratio (NLR) and therapeutic response has been reported as a typical marker in peripheral blood in several cancer types including RCC [6,7,8]. Recently, several studies reported that peripheral eosinophil counts may predict the incidence of irAE and are associated with the response to ICIs in several types of cancer [9,10,11,12,13,14,15,16]. However, there have been not fully understood the association between eosinophil counts and clinical prognosis in RCC, especially with ICIs treatment.
In the present study, we demonstrated that metastatic RCC (mRCC) patients who experienced severe irAE had consistently higher eosinophil counts before and at any time point after the nivolumab when compared to those without. Furthermore, the high absolute eosinophil counts (AECs) at an early time point were significantly associated with a better clinical outcome in mRCC patients. Collectively, these markers may concisely and practically predict clinical response in mRCC patients with anti-PD-1 inhibitor.
Materials and methods
Patients
We retrospectively investigated data from 83 mRCC patients treated by nivolumab at 2 institutions (Osaka International Cancer Institute and Osaka University Medical Hospital) from February 2016 to May 2022. For each patient, we collected baseline demographic and clinical data including age, gender, with or without nephrectomy, histological type, Karnofsky Performance Status (KPS), International Metastatic RCC Database Consortium (IMDC) risk classification, metastatic site and treatment line of nivolumab. Peripheral blood AECs were measured at baseline, 2, 4 and 6 weeks after initiation of nivolumab.
Tumor response was evaluated every 8–12 weeks, according to the Response Evaluation Criteria in Solid Tumors version 1.1 (RECIST ver1.1), using computed tomography. Adverse events were evaluated by Common Terminology Criteria for Adverse Events version 5.0 (CTCAE ver5.0). For each patient, the best response during treatment including complete response (CR), partial response (PR), stable disease (SD) or progressive disease (PD) was measured. Progression-free survival (PFS) was defined as the time from the initiation of nivolumab to documented progression or death of any cause. Overall survival (OS) was defined as the time from the start of nivolumab to documented death of any cause or last contact.
The study was approved by the Institutional Review Board of each institution (approval number 018–0003 in Osaka University Hospital and 18,042 in Osaka International Cancer Institute) and was conducted in accordance with the Declaration of Helsinki.
Treatment
The patients received 240 mg/body of nivolumab every two weeks or 480 mg/body every four weeks until disease progression, clinical deterioration, unacceptable toxicity, or patient’s refusal.
Statistical analysis
We divide the patients into two groups according to the best response and irAE grades. The AECs of two groups were compared using the t test. PFS and OS were estimated by the Kaplan–Meier method and compared with the log-rank test. The prognostic significance of certain parameters was assessed by the Cox proportional hazards regression model. Differences were considered significant at p value < 0.05. All statistical analyses were conducted in JMP-software ver. 17.0 (SAS Institute, Cary, NC, USA).
Results
Patients’ characteristics
The clinical characteristics of all patients are shown in Table 1. The median age at treatment was 64 years (range, 27–83). Nephrectomy had been performed in 74 patients (89%). The most predominant histological type was clear cell RCC (82.0%). With respect to the IMDC risk classification, 7, 67, and 25% were classified as favorable-, intermediate-, and poor-risk, respectively. The treatment line of nivolumab was 2nd, 3rd and 4th or later in 35, 28 and 20 patients, respectively.
Clinical outcomes of patients with nivolumab
The median duration of nivolumab treatment was 5.6 month (range, 0.7–35.7), and the median follow-up was 19.6 month (range, 0.8–59.1). During the observational period, 39 patients died from cancer. Overall, the median OS was 25.5 months, and the median PFS was 5.7 months (Supplementary Fig. 1). Among 83 patients, CR and PR were achieved in 7 (8.4%) and 17 patients (20.5%), respectively, and SD was observed in 30 patients, resulting in objective response rate (ORR) of 28.9% and disease control rate (DCR) of 65.0%. In the present study, we defined responders as patients with CR, PR.
Safety analysis
In the present study, 22 patients (27%) experienced a total of 11 different irAE categories (Table 2). Among them, grade 3 irAE occurred in 13 patients (16%). No patient experienced a grade 4 or higher AE. Of the grade 3 cases, 9 cases improved with nivolumab discontinuation and steroid treatment, whereas 3 cases with adrenal insufficiency required continuous hydrocortisone medication. In addition, 1 case with diabetes mellitus required permanent insulin administration. These 4 patients resumed nivolumab administration after the recovery from irAE.
Correlation between the absolute eosinophil counts (AECs) value and irAE
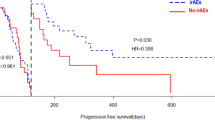
Next, we examined the whether the AECs value affected the grade of irAE. The median AECs value of the patients with irAE at baseline and 4 weeks after the initiation of treatment was significantly higher than those without irAE (217 cells/uL versus 170 cells/uL, p < 0.001 and 357 cells/uL versus 211 cells/uL, p = 0.001, Fig. 1A). The optimal cutoff value of AECs to differentiate the occurrence of irAE was 329 cells/µL at 4 weeks after the treatment, as determined by receiver operating characteristics (ROC) curve (Fig. 1B). The ROC curve was constructed based on the relationship between the occurrence of irAE and the AECs at week 4. According to this ROC curve, the AECs with the highest sensitivity and specificity were determined to be 329 cells/µL. When we divided the patients with the cutoff value (329 cells/µL) of AECs, we observed that the incidence of irAEs was significantly higher in high-AECs group (n = 12, 52%) compared with that in low-AECs group (n = 10, 17%) (p < 0.001, Fig. 1C). Moreover, when we examined the contribution of the irAE to improve the prognosis in mRCC patients, PFS and OS were significantly longer for patients who developed irAE than those who did not (p = 0.021 and p = 0.007, respectively, Fig. 2A and B).
Comparison of absolute eosinophil counts between irAE group and non-irAE group. A The median absolute eosinophil counts (AECs) of patients with irAE at baseline and 4 weeks after the initiation of treatment were significantly higher than those without irAE (217 cells/uL versus 170 cells/uL, p < 0.001 and 357 cells/uL versus 211 cells/uL, p = 0.001). The median value is represented by the middle horizontal line in each box. The bottom and top of each box indicate the 25th and 75th percentiles, respectively. The ends of the whiskers indicate the minimum and maximum of all data. B Receiver operating characteristics curve analysis at 4 weeks after the treatment: area under curve = 0.686, sensitivity = 82.0%, specificity = 59.1%, cutoff value = 329 cells/µL. C When we divided the patients with the cutoff value (329 cells/µL) of counts, we observed that the incidence of irAE was significantly higher in high-AECs group (n = 12, 52%) compared with that in low-AECs group (n = 10, 17%) (p < 0.001)
Correlation between the presence of irAEs and clinical outcomes. Kaplan–Meier survival curves show A progression-free survival (PFS) and B overall survival (OS) between irAE group and non-irAE group. Patients with irAE had significantly better PFS (median 10.9 months versus 3.7 months, p = 0.02) and OS (median NR versus 16.0 months, p = 0.007) than those without irAE
Correlation between the absolute eosinophil counts (AECs) value and clinical efficacy in patients with nivolumab
We further investigated whether the AECs value affects the clinical efficacy of mRCC patients with nivolumab. As a result, responders exhibited a significantly higher AECs value than those of non-responders at 4 weeks after the treatment (242 cells/uL versus 178 cells/uL, p = 0.008, Fig. 3A). Importantly, when we divided the patients with the cutoff value (329 cells/µL), the percentage of responder was significantly higher in high-AECs group compared with that in low-AECs group (p < 0.001, Fig. 3B). The PFS and OS were significantly longer in patients with high-AECs value than those with low-AECs value (p = 0.03 and p = 0.009, respectively, Fig. 4).
Comparison of absolute eosinophil counts between responders and non-responders. A Responders had significantly higher absolute eosinophil counts (AECs) at 4 weeks after the initiation of nivolumab compared to that in non-responders (p < 0.001). B When we divided the patients with the cutoff value (329 cells/µL) of AECs at 4 weeks after the initiation of nivolumab, the percentage of responder was significantly higher in high-AECs group (n = 11, 48%) compared with that in low-AECs group (n = 13, 22%) (p < 0.001)
Correlation between absolute eosinophil counts at 4 weeks after the initiation of nivolumab and clinical outcomes. Kaplan–Meier survival curves show A progression-free survival (PFS) and B overall survival (OS) between high absolute eosinophil counts (AECs) group and low-AECs group. Patients with high-AECs had significantly better PFS (median 12.2 months versus 4.3 months, p = 0.03) and OS (median NR versus 16.4 months, p = 0.009) than those with low-AECs
Furthermore, the impacts of several clinicopathologic factors on PFS and OS in these 83 patients were evaluated (Table 3, 4). Univariate analysis identified AECs value, IMDC classification, the number of metastatic site and irAE were significantly associated with OS. Interestingly, in the multivariate analysis, higher AECs value was significantly associated with a better OS in patients (HR 0.401, 95% CI 0.176–0.909, p = 0.028).
Discussion
The landscape of oncology has been drastically revolutionized by the emergence of ICIs, significantly enhancing the prognosis of previously incurable cancers [17]. However, the response rate to ICIs for mRCC is still limited [18,19,20,21,22], and treatment-related irAE often causes discontinuation of ICIs. Therefore, it is critical to identify biomarkers to predict the response of patients to ICIs to enable a precision medicine approach. Although the association between peripheral eosinophil counts and the response to ICIs has been observed in several types of cancer, the evidence is still limited due to the variety of evaluation methods of eosinophils. Hence, in this study, we performed the data analysis of mRCC patients treated with nivolumab and clarified some evidences about AECs affecting adverse events and clinical outcomes.
First, we found mRCC patients with irAE had higher AECs value compared to those without irAE at baseline and 4 weeks after treatment (Fig. 1A). Our findings partially align with the data published by Ma et al. reported that irAE was more likely to occur in patients with higher AECs at the start of treatment in multiple cancer types treated with PD-1 or PD-L1 inhibitors [15]. Giommoni et al. also reported that high-AECs at the start of treatment were a significant risk factor for the occurrence of irAE in 168 cancer patients including 43 RCC patients [16]. In the present study, we first confirmed continuous activation of eosinophils in patients with irAE throughout nivolumab treatment, suggesting that it may be useful to prepare for the occurrence of irAE by regularly monitoring AECs.
Secondly, our results demonstrated superior efficacy outcomes in patients with high-AECs value at 4 weeks after treatment, as evidenced by improved PFS and OS in this group (Fig. 3, 4). In this decade, several studies reported the relationship between eosinophils and the efficacy of ICI treatment in several types of cancer with a focus on the ratio of pre- and post-treatment eosinophils [9,10,11,12,13]. In our study, we first propose that absolute value of eosinophils counts at 4 weeks after the treatment clearly allows identifying early surrogate biomarkers in the peripheral blood for predicting clinical responses to anti-PD-1 therapy. Eosinophils infiltrate multiple tumors and regulate tumor progression either directly by interacting with tumor cells or indirectly by shaping the tumor microenvironment [23]. Carretero et al. reported that eosinophils evoke further immune response with CD8 + T cells in tumor microenvironment by producing C–C motif chemokine ligand 5 (CCL5), C–X–C motif chemokine ligand 9 (CXCL9), and CXCL10 [24]. These results may support the potential mechanism of anti-cancer immune response of peripheral eosinophils in cancer patients.
There are several limitations to this study. First, our small sample size may limit the generalization for our findings to other types of cancer. Second, we only enrolled mRCC patients with anti-PD-1 therapy. Given that ICI combination therapies have become standard first-line treatments in mRCC field, further prospective studies are required to consolidate our results.
Conclusion
We found that the high absolute eosinophil counts at 4 weeks after the start of nivolumab were a prominent prognostic marker associated with clinical outcome in mRCC patients.
References
Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2021. CA Cancer J Clin. 2021;71(1):7–33.
Cancer facts and figures. [https://www.cancer.org/research/cancer-facts-statistics/all-cancer-facts-figures/cancer-facts-figures-2022.html]
Ljungberg B, Albiges L, Abu-Ghanem Y, Bedke J, Capitanio U, Dabestani S, Fernandez-Pello S, Giles RH, Hofmann F, Hora M, et al. European association of urology guidelines on renal cell carcinoma: the 2022 update. Eur Urol. 2022;82(4):399–410.
Pichler M, Hutterer GC, Chromecki TF, Jesche J, Kampel-Kettner K, Rehak P, Pummer K, Zigeuner R. External validation of the Leibovich prognosis score for nonmetastatic clear cell renal cell carcinoma at a single European center applying routine pathology. J Urol. 2011;186(5):1773–7.
Guida A, Sabbatini R, Gibellini L, De Biasi S, Cossarizza A, Porta C. Finding predictive factors for immunotherapy in metastatic renal-cell carcinoma: what are we looking for? Cancer Treat Rev. 2021;94:102157.
Wu X, Han R, Zhong Y, Weng N, Zhang A. Post treatment NLR is a predictor of response to immune checkpoint inhibitor therapy in patients with esophageal squamous cell carcinoma. Cancer Cell Int. 2021;21(1):356.
Ren F, Zhao T, Liu B, Pan L. Neutrophil-lymphocyte ratio (NLR) predicted prognosis for advanced non-small-cell lung cancer (NSCLC) patients who received immune checkpoint blockade (ICB). Onco Targets Ther. 2019;12:4235–44.
Chen X, Meng F, Jiang R. Neutrophil-to-lymphocyte ratio as a prognostic biomarker for patients with metastatic renal cell carcinoma treated with immune checkpoint inhibitors: a systematic review and meta-analysis. Front Oncol. 2021;11:746976.
Delyon J, Mateus C, Lefeuvre D, Lanoy E, Zitvogel L, Chaput N, Roy S, Eggermont AM, Routier E, Robert C. Experience in daily practice with ipilimumab for the treatment of patients with metastatic melanoma: an early increase in lymphocyte and eosinophil counts is associated with improved survival. Ann Oncol. 2013;24(6):1697–703.
Moreira A, Leisgang W, Schuler G, Heinzerling L. Eosinophilic count as a biomarker for prognosis of melanoma patients and its importance in the response to immunotherapy. Immunotherapy. 2017;9(2):115–21.
Nishikawa D, Suzuki H, Beppu S, Terada H, Sawabe M, Kadowaki S, Sone M, Hanai N. Eosinophil prognostic scores for patients with head and neck squamous cell carcinoma treated with nivolumab. Cancer Sci. 2021;112(1):339–46.
Ghebeh H, Elshenawy MA, AlSayed AD, Al-Tweigeri T. Peripheral blood eosinophil count is associated with response to chemoimmunotherapy in metastatic triple-negative breast cancer. Immunotherapy. 2022;14(4):189–99.
Chu X, Zhao J, Zhou J, Zhou F, Jiang T, Jiang S, Sun X, You X, Wu F, Ren S, et al. Association of baseline peripheral-blood eosinophil count with immune checkpoint inhibitor-related pneumonitis and clinical outcomes in patients with non-small cell lung cancer receiving immune checkpoint inhibitors. Lung Cancer. 2020;150:76–82.
Herrmann T, Ginzac A, Molnar I, Bailly S, Durando X, Mahammedi H. Eosinophil counts as a relevant prognostic marker for response to nivolumab in the management of renal cell carcinoma: a retrospective study. Cancer Med. 2021;10(19):6705–13.
Ma Y, Ma X, Wang J, Wu S, Wang J, Cao B. Absolute eosinophil count may be an optimal peripheral blood marker to identify the risk of immune-related adverse events in advanced malignant tumors treated with PD-1/PD-L1 inhibitors: a retrospective analysis. World J Surg Oncol. 2022;20(1):242.
Giommoni E, Giorgione R, Paderi A, Pellegrini E, Gambale E, Marini A, Antonuzzo A, Marconcini R, Roviello G, Matucci-Cerinic M, et al. Eosinophil count as predictive biomarker of immune-related adverse events (irAEs) in immune checkpoint inhibitors (ICIs) therapies in oncological patients. Immuno. 2021;1(3):253–63.
Ribas A, Wolchok JD. Cancer immunotherapy using checkpoint blockade. Science. 2018;359(6382):1350–5.
Motzer RJ, Escudier B, George S, Hammers HJ, Srinivas S, Tykodi SS, Sosman JA, Plimack ER, Procopio G, McDermott DF, et al. Nivolumab versus everolimus in patients with advanced renal cell carcinoma: Updated results with long-term follow-up of the randomized, open-label, phase 3 CheckMate 025 trial. Cancer. 2020;126(18):4156–67.
Albiges L, Tannir NM, Burotto M, McDermott D, Plimack ER, Barthelemy P, Porta C, Powles T, Donskov F, George S, et al. Nivolumab plus ipilimumab versus sunitinib for first-line treatment of advanced renal cell carcinoma: extended 4-year follow-up of the phase III CheckMate 214 trial. ESMO Open. 2020;5(6):e001079.
Powles T, Plimack ER, Soulieres D, Waddell T, Stus V, Gafanov R, Nosov D, Pouliot F, Melichar B, Vynnychenko I, et al. Pembrolizumab plus axitinib versus sunitinib monotherapy as first-line treatment of advanced renal cell carcinoma (KEYNOTE-426): extended follow-up from a randomised, open-label, phase 3 trial. Lancet Oncol. 2020;21(12):1563–73.
Motzer R, Alekseev B, Rha SY, Porta C, Eto M, Powles T, Grunwald V, Hutson TE, Kopyltsov E, Mendez-Vidal MJ, et al. Lenvatinib plus pembrolizumab or everolimus for advanced renal cell carcinoma. N Engl J Med. 2021;384(14):1289–300.
Choueiri TK, Powles T, Burotto M, Escudier B, Bourlon MT, Zurawski B, Oyervides Juarez VM, Hsieh JJ, Basso U, Shah AY, et al. Nivolumab plus cabozantinib versus sunitinib for advanced renal-cell carcinoma. N Engl J Med. 2021;384(9):829–41.
Grisaru-Tal S, Itan M, Klion AD, Munitz A. A new dawn for eosinophils in the tumour microenvironment. Nat Rev Cancer. 2020;20(10):594–607.
Carretero R, Sektioglu IM, Garbi N, Salgado OC, Beckhove P, Hammerling GJ. Eosinophils orchestrate cancer rejection by normalizing tumor vessels and enhancing infiltration of CD8(+) T cells. Nat Immunol. 2015;16(6):609–17.
Funding
Open Access funding provided by Osaka University. Not applicable.
Author information
Authors and Affiliations
Contributions
A.Y. and A.N. collected the data. A.Y. and T.K. wrote the main manuscript text and prepared figures. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Ethics approval
The study was conducted in accordance with the principles outlined in the Declaration of Helsinki. Approval was obtained from the Medical Ethics Committee of each participating institution (approval number 018–0003 in Osaka University Hospital and 18042 in Osaka International Cancer Institute).
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Retrospectively registered on May 1, 2022: Number 018–0003 in Osaka University Hospital and 18042 in Osaka International Cancer Institute.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Yoshimura, A., Nagahara, A., Ishizuya, Y. et al. The prognostic impact of peripheral blood eosinophil counts in metastatic renal cell carcinoma patients treated with nivolumab. Clin Exp Med 24, 111 (2024). https://doi.org/10.1007/s10238-024-01370-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10238-024-01370-8