Abstract
Background: The incidence of malignant tumors has increased in patients with non-paraneoplastic pemphigus, although there has been no systematic analysis of global epidemiology. Objective: To explore the epidemiology of various types of non-paraneoplastic pemphigus associated with malignant tumors. Methods: Five databases from establishment through October 20, 2023, were searched. STATA SE 17 was used for the data analysis. Subgroup, meta-regression, and sensitivity analyses were used to evaluate the heterogeneity of pooled studies. Results: A total of 6679 participants were included in our meta-analysis from 16 studies. The aggregated prevalence of tumors in patients diagnosed with pemphigus was 8%. The prevalence was 7% in patients with pemphigus vulgaris, 10% in those with pemphigus foliaceus, and 12% in individuals diagnosed with other types of pemphigus. The prevalence was 8% in Asia, 11% in Europe, and 8% in North America. From a country-specific perspective, patients with pemphigus from Israel, Greece, and Germany exhibited a higher prevalence of tumors at 11%. Furthermore, when categorized by the duration of the study period, the highest prevalence was observed in studies spanning 10 to 20 years, at 11%. Conclusion: These findings demonstrate the incidence and prevalence of malignant tumors in patients with non-paraneoplastic pemphigus, which may achieve early detection and intervention, and then reduce mortality rates.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Pemphigus is a severe autoimmune skin disease characterized by chronic, recurrent blisters on the skin or mucous membranes. The primary pathogenesis is believed to be caused by the loss of intercellular adhesion due to autoantibodies targeting the desmoglein 1 and/or desmoglein 3, which are glycoproteins in the cadherin family that facilitate cell–cell adhesion in keratinocytes. It is typically classified into common, proliferative, deciduous, erythematous, and particular types. Its clinical features include the emergence of lax, thin-walled blisters, bullae, and erosions on the skin and mucosa. The overall estimated incidence was 7.2 per million inhabitants per year in Israel [1]. In Germany, it has been determined to be 1.5 per million per year [2]. Data from the 2002–2012 Nationwide Inpatient Sample showed the total annual inpatient cost-of-care for patients admitted with a primary diagnosis of pemphigus was $74,466,305 in America [3].
Systemic application of glucocorticoids is a first-line therapeutic drug. In addition, it is suggested that immunosuppressive agents should be used early in moderate and severe patients [4, 5]. The biological agent rituximab is usually used as a combination of systemic glucocorticoids and can also be used in combination with intravenous immunoglobulin (IVIG) [6]. Other therapies are plasma exchange, immune adsorption, and stem cell transplantation [7]. However, adverse reactions cannot be ignored. Long-term use of glucocorticoids and immunosuppressants can easily lead to infection, even leading to tumors. Patients with severe disease involving the skin and mucosa extensively across the body are susceptible to complications like hypoproteinemia and sepsis, which are life-threatening [8]. Paraneoplastic pemphigus (PNP), currently known to be associated with tumors, is a rare variant of pemphigus, accounting for roughly 5% of all cases. Almost all PNP patients have an association with tumors, benign or malignant [9]. Moreover, instances of other types of pemphigus occurring alongside malignant tumors should not be neglected.
However, there is currently no systematic epidemiological study on pemphigus combined with malignant tumors. Our purpose is to assess the epidemiology of various types of pemphigus associated with malignant tumors to achieve early detection and intervention and then reduce mortality rates.
Methods
This study adhered to the PRISMA 2020 statement (Table S1) and the Meta-analysis of Observational Studies in Epidemiology (MOOSE) guidelines (Table S2).
Search strategy
Two authors independently conducted a search of the PubMed, Embase, CNKI, Wanfang, and Sinomed databases from their establishment until October 20, 2023. The search strategy used both subject terms and free-word combinations. The primary search terms included “pemphigus,” “neoplasms,” “tumor,” “neoplasia,” “cancer,” and “malignancy.”
Study selection
The criteria for inclusion in this study were as follows: (1) studies reporting the prevalence and/or incidence of comorbid tumors in pemphigus patients; (2) observational studies, including cohort, case–control, cross-sectional, and retrospective studies; (3) studies that had received ethics committee approval and had obtained signed informed consent from participants; (4) studies published in either Chinese or English; and (5) studies with no restrictions on sex, age, or country.
The exclusion criteria were: (1) duplicate studies; (2) non-observational studies; (3) studies with unavailable data; and (4) studies for which the full text was not accessible, making it impossible to ascertain whether they met the inclusion criteria.
Data extraction
Two researchers independently extracted the following data: first author’s name, year of publication, period of study, study type, region (country), types of pemphigus, types of tumors, number of pemphigus cases with co-occurring tumors, and prevalence and/or incidence of comorbidities.
Quality assessment
Two authors independently evaluated the pooled studies. For cross-sectional studies, the Agency for Healthcare Research and Quality (AHRQ) tool was employed to assess the risk of bias. For case–control and cohort studies, the Newcastle–Ottawa Scale (NOS) was used for evaluation. Each study was assigned a specific score on the following scale: a score of 0–3 was defined as low quality, 4–7 as medium quality, and 8–11 as high quality.
Statistical analysis
Data analysis was performed using STATA SE 17. Heterogeneity was assessed using the I2 test. If the I2 value was less than 50%, a fixed-effects model was applied; if not, a random-effects model was utilized. Heterogeneity was further addressed through subgroup, meta-regression, and sensitivity analyses. To determine the presence of publication bias, both Egger’s and Begg’s linear regression tests were employed. If publication bias was detected, the trim-and-fill method was enacted to estimate the number of potentially missing studies and correct for the bias.
Results
Characteristics of the included studies
The initial literature search yielded 4,911 studies. After removing duplicates, the titles and abstracts of the remaining 4465 studies were reviewed for relevance. This resulted in 481 studies being chosen for a more detailed, full-text evaluation. However, 465 of these studies were subsequently excluded due to the lack of clear diagnostic criteria, unspecified research methodology, incomplete data, or mismatch with the study topic. In the end, 16 studies (6679 participants) satisfied the inclusion criteria and were included in this meta-analysis (Fig. 1 and Table 1).
Study quality
There was no suitable evaluation instrument for the retrospective studies, so these were excluded from the quality assessments [10,11,12,13,14,15,16,17]. Two studies were classified as high quality [18, 19], while the remaining studies [20,21,22,23,24,25] were given a medium-quality rating. The details of the quality evaluation can be found in Supplementary Tables S3 and S4.
Outcomes
Prevalence
The overall prevalence of tumors in patients diagnosed with pemphigus was found to be 8% (95% CI: 0.07, 0.10) (Fig. 2). A subgroup analysis was conducted, segregated by different types of pemphigus. The prevalence in patients with pemphigus vulgaris was 7% (95% CI: 0.05, 0.10), compared to 10% (95% CI: 0.05, 0.14) in those with pemphigus foliaceus, and 12% (95% CI: 0.02, 0.22) in individuals diagnosed with other types of pemphigus (Figure S1).
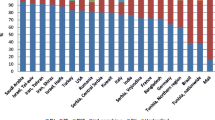
The prevalence of malignancy in patients with pemphigus was reported solely by studies conducted in Asia, Europe, and North America. In terms of continental distribution, the prevalence was 8% (95% CI: 0.05, 0.10) in Asia, 11% (95% CI: 0.09, 0.13) in Europe, and 8% (95% CI: 0.03, 0.12) in North America (Figure S2). Within Asia, six countries reported the following prevalence rates: China at 8% (95% CI: 0.03, 0.13); Japan at 7% (95% CI: 0.01, 0.13); Singapore at 4% (95% CI: − 0.01, 0.10); Turkey at 4% (95% CI: − 0.04, 0.11); Israel at 11% (95% CI: 0.06, 0.16); and Saudi Arabia at 7% (95% CI: 0.01, 0.12). In Europe, four countries reported these figures: Greece at 11% (95% CI: 0.01, 0.21); Bulgaria at 9% (95% CI: 0.03, 0.16); Germany at 11% (95% CI: 0.09, 0.13); and France at 10% (95% CI: 0.06, 0.13). In North America, Canada, and the United States of America (USA) reported comorbid malignancy and pemphigus prevalences of 8% (95% CI: 0.05, 0.11) and 10% (95% CI: 0.01, 0.18), respectively (Figure S3).
Furthermore, the prevalence varied based on the type and duration of the study. In cohort studies, the prevalence was recorded at 6% (95% CI: 0.04, 0.08); in cross-sectional studies, it was 8% (95% CI: 0.04, 0.13); and in retrospective studies, it was 10% (95% CI: 0.08, 0.12) (Figure S4). Additionally, when sorted by the duration of the study period, the prevalence in studies lasting fewer than 10 years was 7% (95% CI: 0.05, 0.09); in studies spanning 10 to 20 years, it was 11% (95% CI: 0.08, 0.14); and in those that extended beyond 20 years, it was 9% (95% CI: 0.06, 0.12) (Figure S5).
Heterogeneity analysis
The Galbraith plot shows that some studies fall outside the confidence interval boundaries, and the steep gradient of the scatter plot indicates existing heterogeneity (Fig. 3).
A meta-regression analysis was performed to identify potential sources of this heterogeneity in prevalence. Unexpectedly, factors such as study design, study quality, geographical region, study duration, and publication year were not identified as sources of heterogeneity in the prevalence of malignancy among patients with pemphigus (Table S5). Nevertheless, subgroup analyses were conducted to address the observed heterogeneity.
Sensitivity analyses
Separate sensitivity analyses were performed to investigate the prevalence of malignancy in patients with pemphigus. The leave-one-out meta-analysis indicates that the findings are both stable and reliable (Fig. 4).
Publication bias
By employing Egger’s and Begg’s linear regression tests, we assessed the potential for publication bias concerning the prevalence of malignancy in patients with pemphigus. Both linear regression tests revealed no evidence of publication bias in relation to malignancy prevalence among patients with pemphigus (Egger test, P = 0.4905; Begg’s test, P = 0.6204).
Discussion
This study scrutinized the global epidemiology of pemphigus and tumors. Overall, the aggregated prevalence of tumors in patients diagnosed with pemphigus was 8%. The prevalence was 7% in patients with pemphigus vulgaris, 10% in those with pemphigus foliaceus, and 12% in individuals diagnosed with other types of pemphigus. The prevalence was 8% in Asia, 11% in Europe, and 8% in North America. From a country-specific perspective, patients with pemphigus from Israel, Greece, and Germany exhibited a higher prevalence of tumors at 11%. Furthermore, when categorized by the duration of the study period, the highest prevalence was observed in studies spanning 10 to 20 years, at 11%. Therefore, it is necessary to strengthen the screening of tumors in patients with pemphigus, especially for those with a disease course of more than 10 years.
The guideline for diagnosis and treatment–guided of pemphigus formulated by the European Dermatology Forum (EDF) in cooperation with the European Academy of Dermatology and Venereology (EADV) have pointed out concerns about pemphigus combined with malignant tumors [4]. For instance, a retrospective study indicated that six out of 96 patients with pemphigus were also diagnosed with malignant tumors, which histopathological and immunopathological evaluations did not reveal the typical traits of paraneoplastic pemphigus [25]. A population-based study between 1985 pemphigus cases and 9847 control individuals from 2004 to 2012 found that the incidence of pemphigus co-occurring with esophageal (OR = 2.9, 95% CI 1.1, 7.4) and laryngeal cancer (OR = 2.0, 95% CI 1.0, 4.1) was significantly higher than in the control group [21]. In a British population-based study using a computerized database, mortality among Pemphigus vulgaris patients was 3.3 times higher than among age- and sex matched control subjects [26]. The medical community and public have acknowledged that malignancy impacts the survival rate in patients with pemphigus [27]. Therefore, early detection, diagnosis, and treatment of potential malignant tumors for patients with pemphigus are of great significance. Our study found that the incidence rate of pemphigus vulgaris and pemphigus foliaceus with malignant tumors was higher in all types, of which the probability of malignant tumors occurring in pemphigus foliaceus was higher than that in pemphigus vulgaris. In a recent German case–control study, PV was found to be associated with oropharyngeal, gastrointestinal, colon neoplasms and hematologic malignancies, whereas PF was associated with nonmelanoma skin cancer [14]. A recent population-based study demonstrated that the prevalence of chronic leukemia, multiple myeloma, and non-Hodgkin lymphoma was greater in patients with pemphigus than in controls [22]. In addition, we found that Israel, Greece, and Germany patients with pemphigus presented a higher prevalence of tumors at 11% (Figure S3). This may be different from the incidence rate of various forms of pemphigus in different countries. Pemphigus vulgaris is the most common in Europe and the USA and has been recognized as the most prevalent type of pemphigus, comprising up to 70% of all cases of pemphigus [28].
There were currently no similar systematic comments published. Our study has some innovative points in several respects. First, we are the only study to date to analyze the epidemiology of malignancy in patients with different types of pemphigus. Second, we systematically analyzed malignancy epidemiology in patients with different types of pemphigus in different countries and regions worldwide. Attention to the incidence of malignant tumors in patients with different types of pemphigus in different regions. For limitation, the comorbidity rates of these two diseases have not been reported in some countries, and larger sample size reports are needed to support our conclusion. However, when we conduct sensitivity analysis, the results are relatively stable.
Conclusion
This is the only study to date to analyze the epidemiology of malignancy in patients with different types of pemphigus. Our findings demonstrate the incidence and prevalence of malignant tumors in patients with non-paraneoplastic pemphigus, which may achieve early detection and intervention, and then reduce mortality rates.
References
Kridin K, Zelber-Sagi S, Khamaisi M, Cohen AD, Bergman R. Remarkable differences in the epidemiology of pemphigus among two ethnic populations in the same geographic region. J Am Acad Dermatol. 2016;75:925–30.
Hahn-Ristic K, Rzany B, Amagai M, Bröcker E, Zillikens D. Increased incidence of pemphigus vulgaris in southern Europeans living in Germany compared with native Germans. J Eur Acad Dermatol Venereol. 2002;16:68–71.
Ren Z, Narla S, Hsu DY, Silverberg JI. Association of serious infections with pemphigus and pemphigoid: analysis of the Nationwide Inpatient Sample. J Eur Acad Dermatol Venereol. 2018;32:1768–76.
Hertl M, Jedlickova H, Karpati S, Marinovic B, Uzun S, Yayli S, et al. Pemphigus. S2 Guideline for diagnosis and treatment–guided by the European Dermatology Forum (EDF) in cooperation with the European Academy of Dermatology and Venereology (EADV). J European Acad Dermatol Venereol. 2015;29(3):405–14.
Eming R, Sticherling M, Hofmann SC, Hunzelmann N, Kern JS, Kramer H, et al. S2k guidelines for the treatment of pemphigus vulgaris/foliaceus and bullous pemphigoid. J Dtsch Dermatol Ges. 2015;13:833–44.
Harman KE, Brown D, Exton LS, Groves RW, Hampton PJ, Mohd Mustapa MF, et al. British association of dermatologists’ guidelines for the management of pemphigus vulgaris 2017. Br J Dermatol. 2017;177:1170–201.
Committee for Guidelines for the Management of Pemphigus Disease, Amagai M, Tanikawa A, Shimizu T, Hashimoto T, Ikeda S, et al. (2014) Japanese guidelines for the management of pemphigus. J Dermatol. 41:471–86
Yamagami J. Recent advances in the understanding and treatment of pemphigus and pemphigoid. F1000Research. 2018;7:1360.
Schmidt E, Kasperkiewicz M, Joly P. Pemphigus. The Lancet. 2019;394:882–94.
Krain LS, Bierman SM. Pemphigus vulgaris and internal malignancy. Cancer. 1974;33:1091–9.
Tsankov N, Vassileva S, Kamarashev J, Kazandjieva J, Kuzeva V. Epidemiology of pemphigus in Sofia, Bulgaria. A 16-year retrospective study (1980–1995). Int J Dermatol. 2000;39(2):104–8.
Goon AT, Tan S. Comparative study of pemphigus vulgaris and pemphigus foliaceus in Singapore. Australas J Dermatol. 2001;42:172–5.
Iwashita K, Matsuyama T, Akasaka E, Mizutani K, Yamamoto K, Kondoh A, et al. The incidence of internal malignancies in autoimmune bullous diseases. Tokai J Exp Clin Med. 2007;32:42–7.
Schulze F, Neumann K, Recke A, Zillikens D, Linder R, Schmidt E. Malignancies in pemphigus and pemphigoid diseases. J Invest Dermatol. 2015;135:1445–7.
Toosi S, Collins JW, Lohse CM, Wolz MM, Wieland CN, Camilleri MJ, Lehman JS. Clinicopathologic features of IgG/IgA pemphigus in comparison with classic (IgG) and IgA pemphigus. Int J Dermatol. 2016;55(4):e184–90. https://doi.org/10.1111/ijd.13025.
Jelti L, Cordel N, Gillibert A, Lacour J-P, Uthurriague C, Doutre M-S, et al. Incidence and mortality of pemphigus in France. J Invest Dermatol. 2019;139:469–73.
Zou H (2023) Analysis of risk factors and comorbidities related to the onset of pemphigus. [Qingdao]: Qingdao University
Akarsu S, Özbağçivan Ö, Dolaş N, Aktan Ş. Possible triggering factors and comorbidities in newlydiagnosed autoimmune bullous diseases. Turk J Med Sci. 2017;47:832–40.
Kridin K, Zelber-Sagi S, Bergman R. Risk factors for lethal outcome in patients with pemphigus: a retrospective cohort study. Eur J Dermatol. 2018;28:26–37.
Heelan K, Mahar AL, Walsh S, Shear NH. Pemphigus and associated comorbidities: a cross-sectional study. Clin Exp Dermatol. 2015;40:593–9.
Kridin K, Zelber-Sagi S, Comaneshter D, Cohen AD. Coexistent solid malignancies in pemphigus: a population-based study. JAMA Dermatol. 2018;154:435.
Kridin K, Zelber-Sagi S, Comaneshter D, Batat E, Cohen AD. Pemphigus and hematologic malignancies: a population-based study of 11,859 patients. J Am Acad Dermatol. 2018;78:1084-1089.e1.
Kyriakis Md KP, Tosca Md AD. Epidemiologic observations on the natural course of pemphigus vulgaris. Int J Dermatol. 1998;37:215–9.
Morioka S, Sakuma M, Ogawa H. The incidence of internal malignancies in autoimmune blistering diseases: pemphigus and bullous pemphigoid in Japan. Dermatology. 1994;189:82–4.
Zeng J, Wang B, Yan Y, Qu T, You J, Yu B. (2003) Clinical analysis of pemphigus, pemphigoid, and dermatomyositis with malignant tumors. J Clin Dermatol. 327–8.
Langan SM, Smeeth L, Hubbard R, Fleming KM, Smith CJP, West J. Bullous pemphigoid and pemphigus vulgaris–incidence and mortality in the UK: population based cohort study. BMJ. 2008;337:a180–a180.
Buonavoglia A, Leone P, Dammacco R, Di Lernia G, Petruzzi M, Bonamonte D, et al. Pemphigus and mucous membrane pemphigoid: an update from diagnosis to therapy. Autoimmun Rev. 2019;18:349–58.
Meyer N, Misery L. Geoepidemiologic considerations of auto-immune pemphigus. Autoimmun Rev. 2010;9:A379–82.
Funding
This study was supported by the Shanghai Dermatology Research Center (2023ZZ02017); Shanghai Dermatology Hospital demonstration research ward project (SHDC2023CRW009); Shanghai Municipal Health Commission Health Industry Clinical Research Special Project (20234Y0269, 20234Y0075, 20224Y0373); Xinglin Youth Scholar of Shanghai University of Traditional Chinese Medicine (RY411.33.10); Youth Talent Promotion Project of China Association of Traditional Chinese Medicine (2021–2023) Category A (CACM-2021-QNRC2-A10); Chen Guang project supported by Shanghai Municipal Education Commission and Shanghai Education Development Foundation (22CGA50); Health Young Talents of Shanghai Municipal Health Commission (2022YQ026); Shanghai Science and Technology Development Funds (Sailing Program) (21YF1441500); Clinical Transformation Incubation Program in Hospital (lcfy2020-06, lczh2021-05, lcfy2022-04, lcfy2022-10, lczh2023-01); Shanghai Science and Technology Committee (21Y21920101, 21Y21920102).
Author information
Authors and Affiliations
Contributions
YL and XF conceived this study. MW, YH, and YZ designed this study. YC, YL, and XD searched the literature and extracted data. QZ and CG assessed the quality of trials and analyzed the data. FS and RW prepared the original manuscript draft. JS and LK contributed to revise the manuscript. All authors have read and approved the final manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
We confirm that there are no known conflicts of interest associated with this publication and there has been no significant financial support for this work that could have influenced its outcome.
Data availability Statement
All data generated in this study are included in this article and its supplementary information files.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Luo, Y., Fei, X., Wang, M. et al. Epidemiology of malignant tumors in patients with pemphigus: an analysis of trends from 1955 to 2021. Clin Exp Med 24, 100 (2024). https://doi.org/10.1007/s10238-024-01354-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10238-024-01354-8