Abstract
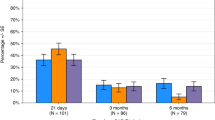
Chimeric antigen receptor T (CAR-T) cell therapy exhibits remarkable efficacy against refractory or relapsed multiple myeloma (RRMM); however, the immune deficiency following CAR-Ts infusion has not been well studied. In this study, 126 patients who achieved remission post-CAR-Ts infusion were evaluated for cellular immunity. Following lymphodepletion (LD) chemotherapy, the absolute lymphocyte count (ALC) and absolute counts of lymphocyte subsets were significantly lower than baseline at D0. Grade ≥ 3 lymphopenia occurred in 99% of patients within the first 30 days, with most being resolved by 180 days. The median CD4+ T-cell count was consistently below baseline and the lower limit of normal (LLN) levels at follow-up. Conversely, the median CD8+ T-cell count returned to the baseline and LLN levels by D30. The median B-cell count remained lower than baseline level at D60 and returned to baseline and LLN levels at D180. In the first 30 days, 27 (21.4%) patients had 29 infections, with the majority being mild to moderate in severity (21/29; 72.4%). After day 30, 44 (34.9%) patients had 56 infections, including 20 severe infections. One patient died from bacteremia at 3.8 months post-CAR-Ts infusion. In conclusion, most patients with RRMM experienced cellular immune deficiency caused by LD chemotherapy and CAR-Ts infusion. The ALC and most lymphocyte subsets gradually recovered after day 30 of CAR-Ts infusion, except for CD4+ T cells. Some patients experience prolonged CD4+ T-cell immunosuppression without severe infection.




Similar content being viewed by others
Data availability
The data presented in this study are available in this article (and Supplementary Materials).
References
Raje N, Berdeja J, Lin Y, et al. Anti-BCMA CAR T-cell therapy bb2121 in relapsed or refractory multiple myeloma. N Engl J Med. 2019;380:1726–37. https://doi.org/10.1056/NEJMoa1817226.
Brudno JN, Maric I, Hartman SD, et al. T cells genetically modified to express an anti-B-cell maturation antigen chimeric antigen receptor cause remissions of poor-prognosis relapsed multiple myeloma. J Clin Oncol. 2018;36:2267–80. https://doi.org/10.1200/JCO.2018.77.8084.
Ali SA, Shi V, Maric I, et al. T cells expressing an anti-B-cell maturation antigen chimeric antigen receptor cause remissions of multiple myeloma. Blood. 2016;128(13):1688–700. https://doi.org/10.1182/blood-2016-04-711903.
Garfall AL, Maus MV, Hwang WT, et al. Chimeric antigen receptor T cells against CD19 for multiple myeloma. N Engl J Med. 2015;373:1040–7. https://doi.org/10.1056/NEJMoa1504542.
Wang Y, Cao J, Gu W, et al. Long-term follow-up of combination of B-cell maturation antigen and CD19 chimeric antigen receptor T cells in multiple myeloma. J Clin Oncol. 2022;40:2246–56. https://doi.org/10.1200/JCO.21.01676.
Lee DW, Santomasso BD, Locke FL, et al. ASTCT consensus grading for cytokine release syndrome and neurologic toxicity associated with immune effector cells. Biol Blood Marrow Transpl. 2019;25:625–38. https://doi.org/10.1016/j.bbmt.2018.12.758.
Xu J, Chen LJ, Yang SS, et al. Exploratory trial of a biepitopic CAR T-targeting B cell maturation antigen in relapsed/refractory multiple myeloma. Proc Natl Acad Sci USA. 2019;116:9543–51. https://doi.org/10.1073/pnas.1819745116.
Zhou D, Wang Y, Cheng H, et al. Factors associated with infection events after chimeric antigen receptor T-cell therapy for relapsed or refractory multiple myeloma. Infect Chemother. 2023;29:179–85. https://doi.org/10.1016/j.jiac.2022.10.012.
Kambhampati S, Sheng Y, Huang CY, et al. Infectious complications in patients with relapsed refractory multiple myeloma after BCMA CAR T-cell therapy. Blood Adv. 2022;6:2045–54. https://doi.org/10.1182/bloodadvances.2020004079.
Neelapu SS, Locke FL, Bartlett NL, et al. Axicabtagene ciloleucel CAR T-cell therapy in refractory large B-cell lymphoma. N Engl J Med. 2017;377:2531–44. https://doi.org/10.1056/NEJMoa1707447.
Jain T, Knezevic A, Pennisi M, et al. Hematopoietic recovery in patients receiving chimeric antigen receptor T-cell therapy for hematologic malignancies. Blood Adv. 2020;4:3776–87. https://doi.org/10.1182/bloodadvances.2020002509.
Hill JA, Li D, Hay KA, et al. Infectious complications of CD19-targeted chimeric antigen receptor-modified T-cell immunotherapy. Blood. 2018;131:121–30. https://doi.org/10.1182/blood-2017-07-793760.
Jain MD, Davila ML. Concise review: emerging principles from the clinical application of chimeric antigen receptor T cell therapies for B cell malignancies. Stem Cells. 2018;36:36–44. https://doi.org/10.1002/stem.2715.
Logue JM, Zucchetti E, Bachmeier CA, et al. Immune reconstitution and associated infections following axicabtagene ciloleucel in relapsed or refractory large B-cell lymphoma. Haematologica. 2021;106:978–86. https://doi.org/10.3324/haematol.2019.238634.
Palumbo A, Rajkumar SV, San Miguel JF, et al. International myeloma working group consensus statement for the management, treatment, and supportive care of patients with myeloma not eligible for standard autologous stem-cell transplantation. J Clin Oncol. 2014;32:587–600. https://doi.org/10.1200/JCO.2013.48.7934.
Pennisi M, Jain T, Santomasso BD, et al. Comparing CAR T-cell toxicity grading systems: application of the ASTCT grading system and implications for management. Blood Adv. 2020;4:676–86. https://doi.org/10.1182/bloodadvances.2019000952.
Bhoj VG, Arhontoulis D, Wertheim G, et al. Persistence of long-lived plasma cells and humoral immunity in individuals responding to CD19-directed CAR T-cell therapy. Blood. 2016;128:360–70. https://doi.org/10.1182/blood-2016-01-694356.
Institute NC. Common terminology criteria for adverse events version 4.03. US Department of Health and Human Services. 2010.
Park JH, Romero FA, Tau Y, et al. Cytokine release syndrome grade as a predictive marker for infections in patients with relapsed or refractory B-cell acute lymphoblastic leukemia treated with chimeric antigen receptor T cells. Clin Infect Dis. 2018;67:533–40. https://doi.org/10.1093/cid/ciy152.
Hansen DK, Sidana S, Peres LC, et al. Idecabtagene vicleucel for relapsed/refractory multiple myeloma: real-world experience from the myeloma CAR T consortium. J Clin Oncol. 2023;41(11):2087–97. https://doi.org/10.1200/JCO.22.01365.
Parikh SA, Leis JF, Chaffee KG, et al. Hypogammaglobulinemia in newly diagnosed chronic lymphocytic leukemia: natural history, clinical correlates, and outcomes. Cancer. 2015;121:2883–91. https://doi.org/10.1002/cncr.29438.
Deyà-Martínez A, Alonso-Saladrigues A, García AP, et al. Kinetics of humoral defificiency in CART19-treated children and young adults with acute lymphoblastic leukaemia. Bone Marrow Transpl. 2021;56:376–86. https://doi.org/10.1038/s41409-020-01027-6.
Cordeiro A, Bezerra ED, Hirayama AV, et al. Late events after treatment with CD19-targeted chimeric antigen receptor modified T cells. Biol Blood Marrow Transpl. 2020;26:26–33. https://doi.org/10.1016/j.bbmt.2019.08.003.
Wang Y, Li C, Xia J, et al. Humoral immune reconstitution after anti-BCMA CAR T-cell therapy in relapsed/refractory multiple myeloma. Blood Adv. 2021;5:5290–9. https://doi.org/10.1182/bloodadvances.2021004603.
Fried S, Avigdor A, Bielorai B, et al. Early and late hematologic toxicity following CD19 CAR-T cells. Bone Marrow Transpl. 2019;54:1643–50. https://doi.org/10.1038/s41409-019-0487-3.
Haneen S, Nirali NS, Terry JF, Bonnie Y, Cynthia D. Chimeric antigen receptor induced cytopenia differs from chemotherapy induced myelosuppression. Blood. 2017;130(Suppl 1):S5048. https://doi.org/10.1182/blood.v130.
Kochenderfer JN, Somerville RPT, Lu T, et al. Long-duration complete remissions of diffuse large B cell lymphoma after anti-CD19 chimeric antigen receptor T cell therapy. Mol Ther. 2017;25:2245–53. https://doi.org/10.1016/j.ymthe.2017.07.004.
Schuster SJ, Svoboda J, Chong EA, et al. Chimeric antigen receptor T cells in refractory B-cell lymphomas. N Engl J Med. 2017;377:2545–54. https://doi.org/10.1056/NEJMoa1708566.
Hay KA, Hanafi LA, Li D, et al. Kinetics and biomarkers of severe cytokine release syndrome after CD19 chimeric antigen receptor-modified T-cell therapy. Blood. 2017;130:2295–306. https://doi.org/10.1182/blood-2017-06-793141.
Cheng J, Mao X, Chen C, et al. Monitoring anti-CD19 chimeric antigen receptor T cell population by flow cytometry and its consistency with digital droplet polymerase chain reaction. Cytometry A. 2023;103(1):16–26. https://doi.org/10.1002/cyto.a.24676.
Cao J, Wang G, Cheng H, et al. Potent anti-leukemia activities of humanized CD19-targeted chimeric antigen receptor T (CAR-T) cells in patients with relapsed/refractory acute lymphoblastic leukemia. Am J Hematol. 2018;93(7):851–8. https://doi.org/10.1002/ajh.25108.
Strati P, Wierda W, Burger J, et al. Myelosuppression after frontline fludarabine, cyclophosphamide, and rituximab in patients with chronic lymphocytic leukemia: analysis of persistent and new-onset cytopenia. Cancer Am Cancer Soc. 2013;119:3805–11. https://doi.org/10.1002/cncr.28318.
Tam CS, O’Brien S, Wierda W, et al. Long-term results of the fludarabine, cyclophos phamide, and rituximab regimen as initial therapy of chronic lymphocytic leukemia. Blood. 2008;112:975–80. https://doi.org/10.1182/blood-2008-02-140582.
Joffe E, Ariela Arad N, Bairey O, et al. Persistently low lymphocyte counts after FCR therapy for chronic lymphocytic leukemia are associated with longer overall survival. Hematol Oncol. 2018;36:128–35. https://doi.org/10.1002/hon.2444.
Ysebaert L, Gross E, Kühlein E, et al. Immune recovery after fludarabine-cyclophosphamide-rituximab treatment in B-chronic lymphocytic leukemia: implication for maintenance immunotherapy. Leukemia. 2010;24:1310–6. https://doi.org/10.1038/leu.2010.89.
Baird JH, Epstein DJ, Tamaresis JS, et al. Immune reconstitution and infectious complications following axicabtagene ciloleucel therapy for large B-cell lymphoma. Blood Adv. 2021;5:143–55. https://doi.org/10.1182/bloodadvances.2020002732.
Wang Y, Li H, Song X, et al. Kinetics of immune reconstitution after anti-CD19 chimeric antigen receptor T cell therapy in relapsed or refractory acute lymphoblastic leukemia patients. Int J Lab Hematol. 2021;43:250–8. https://doi.org/10.1111/ijlh.13375.
Logue JM, Zucchetti E, Bachmeier CA, et al. Immune reconstitution and associated infections following axicabtagene ciloleucel in relapsed or refractory large B-cell lymphoma. Haematologica. 2020;106:978–86. https://doi.org/10.3324/haematol.2019.238634.
Laurent J, Speiser DE, Appay V, et al. Impact of 3 different short- term chemotherapy regimens on lymphocyte- depletion and reconstitution in melanoma patients. J Immunother. 2010;33:723–34. https://doi.org/10.1097/CJI.0b013e3181ea7e6e.
Mackall CL, Fleisher TA, Brown MR, et al. Age, thymopoiesis, and CD4+ T-lymphocyte regeneration after intensive chemotherapy. N Engl J Med. 1995;332:143–9. https://doi.org/10.1056/NEJM199501193320303.
Mackall CL, Fleisher TA, Brown MR, et al. Distinctions between CD8+ and CD4+ T-cell regenerative pathways result in prolonged T-cell subset imbalance after intensive chemotherapy. Blood. 1997;89:3700–7. https://doi.org/10.1182/blood.v89.10.3700.
Keating MJ, O’Brien S, Lerner S, et al. Long-term follow-up of patients with chronic lymphocytic leukemia (CLL) receiving fludarabine regimens as initial therapy. Blood. 1998;92:1165–71. https://doi.org/10.1182/blood.v92.4.1165.
Bouaziz JD, Yanaba K, Venturi GM, et al. Therapeutic B cell depletion impairs adaptive and autoreactive CD4+ T cell activation in mice. Proc Natl Acad Sci USA. 2007;104(52):20878–83. https://doi.org/10.1073/pnas.0709205105.
Lee DW, Kochenderfer JN, Stetler-Stevenson M, et al. T cells expressing CD19 chimeric antigen receptors for acute lymphoblastic leukaemia in children and young adults: a phase 1 dose-escalation trial. Lancet. 2015;385(9967):517–28. https://doi.org/10.1016/S0140-6736(14)61403-3.
Maude SL, Laetsch TW, Buechner J, et al. Tisagenlecleucel in children and young adults with B-cell lymphoblastic leukemia. N Engl J Med. 2018;378(5):439–48. https://doi.org/10.1056/NEJMoa1709866.
Jung AC, Paauw DS. Diagnosing HIV-related disease: using the CD4 count as a guide. J Gen Intern Med. 1998;13:131–6. https://doi.org/10.1046/j.1525-1497.1998.00031.x.
Telli Dizman G, Aguado JM, Fernández-Ruiz M. Risk of infection in patients with hematological malignancies receiving CAR T-cell therapy: systematic review and meta-analysis. Expert Rev Anti Infect Ther. 2022;20(11):1455–76. https://doi.org/10.1080/14787210.2022.2128762.
Yan ZM, Liu YQ, Huang ZF, et al. The Value of T Cell subsets and Cytokine Levels Changes in the Clinical Diagnosis, Treatment and Prognosis Evaluation of Multiple Myeloma. Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2022;30(6):1791–6. https://doi.org/10.19746/j.cnki.issn.1009-2137.2022.06.025.
Funding
This work was financially supported by Grants from the Key Research & Development Plan of Jiangsu Province (BE2018634, BE2020681, BE2022711), Xuzhou Medical leading talents Training Program (XWRCHT20210028).
Author information
Authors and Affiliations
Contributions
HC and JC designed and supervised the study; XW, WC, WS, KQ, ZL, CS, FX, HH and WG and their respective research teams recruited and followed up with the patients and provided clinical data; SJ, JW, TH, ZC, JL and LS collected and analyzed the research data; HC, SJ, JW, TH and JC performed statistical analyses; SJ, JW, TH, ZC, JL, LS, XW, WS and KQ took care of the patients and evaluated responses; HC, SJ and JC drafted and revised the manuscript; MS, JQ, QW and LZ contributed to laboratory monitoring of the patients; KX, JZ and JC provided support for the project. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Conflicts of interest
The authors declare that they have no conflict of interests.
Ethics approval
The study was approved by the Ethics Committees of the Affiliated Hospital of Xuzhou Medical University, Xuzhou, China (REC ref no. XYFY2020-KL062, XYFY2019-KL182, XYFY2017-KL013).
Consent to participate
All patients provided written informed consent. This study was registered on Chictr.org.cn, number ChiCTR2000033567, ChiCTR-OIC-17011272, ChiCTR1900026219.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Cheng, H., Ji, S., Wang, J. et al. Long-term analysis of cellular immunity in patients with RRMM treated with CAR-T cell therapy. Clin Exp Med 23, 5241–5254 (2023). https://doi.org/10.1007/s10238-023-01232-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10238-023-01232-9