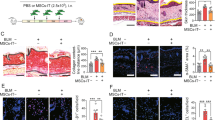
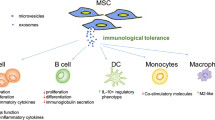
Abstract
Systemic sclerosis (SSc) refers to an autoimmune disease characterized by immune dysfunction, vascular endothelial damage, and multi-organ fibrosis. Thus far, this disease is incurable, and its high mortality rate is significantly correlated with fibrotic events. Fibrosis has been confirmed as a difficult clinical treatment area that should be urgently treated in clinical medicine. Mesenchymal stem cells (MSCs) exhibit immunomodulatory, pro-angiogenic, and anti-fibrotic functions. MSCs-derived extracellular vesicles (EVs) have aroused rising interest as a cellular component that retains the functions of MSCs while circumventing the possible adverse effects of MSCs. Moreover, EVs have great potential in treating SSc. In this study, the current research progress on MSCs and their EVs for treating fibrosis in SSc was reviewed, with an aim to provide some reference for future MSCs and their EVs in treating SSc.

Similar content being viewed by others
Data availability
All data are available from the corresponding author.
Abbreviations
- SSc:
-
Systemic sclerosis
- MSCs:
-
Mesenchymal stem cells
- EVs:
-
Extracellular vesicles
- BMSCs:
-
Bone Marrow-derived stem cells
- AMSCs:
-
Adipose-derived mesenchymal stem cells
- TSG-6:
-
Tumor necrosis factor-stimulated gene 6
- TSG101:
-
Tumor susceptibility gene 101
- HSP:
-
Heat shock protein
- BLM:
-
Bleomycin
- WT:
-
Wild type
- HGF:
-
Hepatocyte growth factor
- LMWH:
-
Low molecular heparin
- HOCl:
-
Hypochlorous acid
- AOPPs:
-
Advanced oxidation protein products
- Tsk:
-
Tight-skin
- GVHD:
-
Chronic graft versus host disease
- MRSS:
-
Modified rodnan skin scores
- HAQ-DI:
-
Health assessment questionnaire disease activity index
- PE:
-
Plasma exchange
- GMP:
-
Good manufacturing practices
References
Denton CP, Khanna D. Systemic sclerosis. Lancet (London, England). 2017;390(10103):1685–99.
Jerjen R, Nikpour M, Krieg T, et al. Systemic sclerosis in adults. Part I: clinical features and pathogenesis. J Am Acad Dermatol. 2022;87(5):937–54.
Bairkdar M, Rossides M, Westerlind H, et al. Incidence and prevalence of systemic sclerosis globally: a comprehensive systematic review and meta-analysis. Rheumatology (Oxford). 2021;60(7):3121–33.
Elhai M, Meune C, Avouac J, et al. Trends in mortality in patients with systemic sclerosis over 40 years: a systematic review and meta-analysis of cohort studies. Rheumatology (Oxford). 2012;51(6):1017–26.
Ramos-Casals M, Fonollosa-Pla V, Brito-Zerón P, et al. Targeted therapy for systemic sclerosis: how close are we? Nat Rev Rheumatol. 2010;6(5):269–78.
Martin-Lopez M, Carreira PE. Antifibrotics in systemic sclerosis. Best Pract Res Clin Rheumatol. 2021;35(3):101671.
Varrica C, Dias HS, Reis C, et al. Targeted delivery in scleroderma fibrosis. Autoimmun Rev. 2021;20(2):102730.
Li A, Guo F, Pan Q, et al. Mesenchymal stem cell therapy: hope for patients with systemic lupus erythematosus. Front Immunol. 2021;12:728190.
Voswinkel J, Francois S, Simon JM, et al. Use of mesenchymal stem cells (MSC) in chronic inflammatory fistulizing and fibrotic diseases: a comprehensive review. Clin Rev Allergy Immunol. 2013;45(2):180–92.
Casiraghi F, Remuzzi G, Abbate M, et al. Multipotent mesenchymal stromal cell therapy and risk of malignancies. Stem Cell Rev Rep. 2013;9(1):65–79.
Rani S, Ryan AE, Griffin MD, et al. Mesenchymal stem cell-derived extracellular vesicles: toward cell-free therapeutic applications. Mol Ther. 2015;23(5):812–23.
Lv FJ, Tuan RS, Cheung KM, et al. Concise review: the surface markers and identity of human mesenchymal stem cells. Stem Cells. 2014;32(6):1408–19.
Galipeau J, Sensébé L. Mesenchymal stromal cells: clinical challenges and therapeutic opportunities. Cell Stem Cell. 2018;22(6):824–33.
Ménard C, Tarte K. Immunoregulatory properties of clinical grade mesenchymal stromal cells: evidence, uncertainties, and clinical application. Stem Cell Res Ther. 2013;4(3):64.
Friedenstein AJ, Gorskaja JF, Kulagina NN. Fibroblast precursors in normal and irradiated mouse hematopoietic organs. Exp Hematol. 1976;4(5):267–74.
Zhou SL, Zheng C, Su JQ, et al. Isolation and identification of human umbilical cord and placenta-derived stem cells and their component analysis. Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2015;23(6):1684–91.
Mebarki M, Abadie C, Larghero J, et al. Human umbilical cord-derived mesenchymal stem/stromal cells: a promising candidate for the development of advanced therapy medicinal products. Stem Cell Res Ther. 2021;12(1):152.
Puissant B, Barreau C, Bourin P, et al. Immunomodulatory effect of human adipose tissue-derived adult stem cells: comparison with bone marrow mesenchymal stem cells. Br J Haematol. 2005;129(1):118–29.
Ménard C, Dulong J, Roulois D, et al. Integrated transcriptomic, phenotypic, and functional study reveals tissue-specific immune properties of mesenchymal stromal cells. Stem Cells. 2020;38(1):146–59.
Loisel S, Dulong J, MéNARD C, et al. Brief report: proteasomal indoleamine 2,3-dioxygenase degradation reduces the immunosuppressive potential of clinical grade-mesenchymal stromal cells undergoing replicative senescence. Stem Cells. 2017;35(5):1431–6.
Shi Y, Wang Y, Li Q, et al. Immunoregulatory mechanisms of mesenchymal stem and stromal cells in inflammatory diseases. Nat Rev Nephrol. 2018;14(8):493–507.
Maumus M, Guérit D, Toupet K, et al. Mesenchymal stem cell-based therapies in regenerative medicine: applications in rheumatology. Stem Cell Res Ther. 2011;2(2):14.
Farge D, Loisel S, Lansiaux P, et al. Mesenchymal stromal cells for systemic sclerosis treatment. Autoimmun Rev. 2021;20(3):102755.
Chen W, Xia ZK, Zhang MH, et al. Adipose tissue-derived stem cells ameliorates dermal fibrosis in a mouse model of scleroderma. Asian Pac J Trop Med. 2017;10(1):52–6.
Yang Y, Zhu S, Li Y, et al. Human umbilical cord mesenchymal stem cells ameliorate skin fibrosis development in a mouse model of bleomycin-induced systemic sclerosis. Exp Ther Med. 2020;20(6):257.
Moroncini G, Paolini C, Orlando F, et al. Mesenchymal stromal cells from human umbilical cord prevent the development of lung fibrosis in immunocompetent mice. PLoS ONE. 2018;13(6):e0196048.
Suzuka T, Kotani T, Saito T, et al. Therapeutic effects of adipose-derived mesenchymal stem/stromal cells with enhanced migration ability and hepatocyte growth factor secretion by low-molecular-weight heparin treatment in bleomycin-induced mouse models of systemic sclerosis. Arthritis Res Ther. 2022;24(1):228.
Jin J, Ou Q, Wang Z, et al. BMSC-derived extracellular vesicles intervened the pathogenic changes of scleroderma in mice through miRNAs. Stem Cell Res Ther. 2021;12(1):327.
Li M, Zhang HP, Wang XY, et al. Mesenchymal stem cell-derived exosomes ameliorate dermal fibrosis in a murine model of bleomycin-induced scleroderma. Stem Cells Dev. 2021;30(19):981–90.
Maria AT, Toupet K, Bony C, et al. Antifibrotic, antioxidant, and immunomodulatory effects of mesenchymal stem cells in HOCl-induced systemic sclerosis. Arthritis Rheumatol (Hoboken, NJ). 2016;68(4):1013–25.
Maria ATJ, Toupet K, Maumus M, et al. Fibrosis development in HOCl-induced systemic sclerosis: a multistage process hampered by mesenchymal stem cells. Front Immunol. 2018;9:2571.
Jin X, Hou J, Zheng K, et al. Umbilical cord mesenchymal stem cells for inhibiting the fibrosis and autoimmune development in HOCl-induced systemic scleroderma mouse model. Int J Stem Cells. 2021;14(3):262–74.
Elessawi DF, Gabr H, Badawy MMM, et al. Therapeutic potential of mesenchymal stem cells for scleroderma induced in mouse model. Tissue Cell. 2021;73:101671.
Maria AT, Toupet K, Maumus M, et al. Human adipose mesenchymal stem cells as potent anti-fibrosis therapy for systemic sclerosis. J Autoimmun. 2016;70:31–9.
Akiyama K, Chen C, Wang D, et al. Mesenchymal-stem-cell-induced immunoregulation involves FAS-ligand-/FAS-mediated T cell apoptosis. Cell Stem Cell. 2012;10(5):544–55.
Chen C, Wang D, Moshaverinia A, et al. Mesenchymal stem cell transplantation in tight-skin mice identifies miR-151-5p as a therapeutic target for systemic sclerosis. Cell Res. 2017;27(4):559–77.
Lim JY, Ryu DB, Lee SE, et al. Mesenchymal stem cells (MSCs) attenuate cutaneous sclerodermatous graft-versus-host disease (Scl-GVHD) through inhibition of immune cell infiltration in a mouse model. J Invest Dermatol. 2017;137(9):1895–904.
Okamura A, Matsushita T, Komuro A, et al. Adipose-derived stromal/stem cells successfully attenuate the fibrosis of scleroderma mouse models. Int J Rheum Dis. 2020;23(2):216–25.
Zhao F, Zhang YF, Liu YG, et al. Therapeutic effects of bone marrow-derived mesenchymal stem cells engraftment on bleomycin-induced lung injury in rats. Transplant Proc. 2008;40(5):1700–5.
Moodley Y, Atienza D, Manuelpillai U, et al. Human umbilical cord mesenchymal stem cells reduce fibrosis of bleomycin-induced lung injury. Am J Pathol. 2009;175(1):303–13.
Wu Y, Huang S, Enhe J, et al. Bone marrow-derived mesenchymal stem cell attenuates skin fibrosis development in mice. Int Wound J. 2014;11(6):701–10.
Yamamoto T, Takagawa S, Katayama I, et al. Animal model of sclerotic skin. I: Local injections of bleomycin induce sclerotic skin mimicking scleroderma. J Invest Dermatol. 1999;112(4):456–62.
Beyer C, Schett G, Distler O, et al. Animal models of systemic sclerosis: prospects and limitations. Arthritis Rheum. 2010;62(10):2831–44.
Cahill EF, Kennelly H, Carty F, et al. Hepatocyte growth factor is required for mesenchymal stromal cell protection against bleomycin-induced pulmonary fibrosis. Stem Cells Transl Med. 2016;5(10):1307–18.
Sakiyama R, Fukuta K, Matsumoto K, et al. Stimulation of hepatocyte growth factor production by heparin-derived oligosaccharides. J Biochem. 2007;141(5):653–60.
Servettaz A, Goulvestre C, Kavian N, et al. Selective oxidation of DNA topoisomerase 1 induces systemic sclerosis in the mouse. J Immunol. 2009;182(9):5855–64.
Green MC, Sweet HO, Bunker LE. Tight-skin, a new mutation of the mouse causing excessive growth of connective tissue and skeleton. Am J Pathol. 1976;82(3):493–512.
Jagasia MH, Greinix HT, Arora M, et al. National institutes of health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: I. The 2014 diagnosis and staging working group report. Biol Blood Marrow Transplant. 2015;21(3):389-401.e1.
Jaffee BD, Claman HN. Chronic graft-versus-host disease (GVHD) as a model for scleroderma. I. Description of model systems. Cell Immunol. 1983;77(1):1–12.
Yang X, Liu C, Fujino M, et al. A modified graft-versus-host-induced model for systemic sclerosis, with pulmonary fibrosis in Rag2-deficient mice. FEBS Open Bio. 2017;7(9):1316–27.
Claman HN, Jaffee BD, Huff JC, et al. Chronic graft-versus-host disease as a model for scleroderma. II. Mast cell depletion with deposition of immunoglobulins in the skin and fibrosis. Cell Immunol. 1985;94(1):73–84.
Christopeit M, Schendel M, FöLL J, et al. Marked improvement of severe progressive systemic sclerosis after transplantation of mesenchymal stem cells from an allogeneic haploidentical-related donor mediated by ligation of CD137L. Leukemia. 2008;22(5):1062–4.
Keyszer G, Christopeit M, Fick S, et al. Treatment of severe progressive systemic sclerosis with transplantation of mesenchymal stromal cells from allogeneic related donors: report of five cases. Arthritis Rheum. 2011;63(8):2540–2.
Guiducci S, Porta F, Saccardi R, et al. Autologous mesenchymal stem cells foster revascularization of ischemic limbs in systemic sclerosis: a case report. Ann Intern Med. 2010;153(10):650–4.
Scuderi N, Ceccarelli S, Onesti MG, et al. Human adipose-derived stromal cells for cell-based therapies in the treatment of systemic sclerosis. Cell Transplant. 2013;22(5):779–95.
Wehbe T, Abi Saab M, Abi Chahine N, et al. Mesenchymal stem cell therapy for refractory scleroderma: a report of 2 cases. Stem Cell Investig. 2016;3:48.
Zhang H, Liang J, Tang X, et al. Sustained benefit from combined plasmapheresis and allogeneic mesenchymal stem cells transplantation therapy in systemic sclerosis. Arthritis Res Ther. 2017;19(1):165.
Wang J, Cai J, Zhang Q, et al. Fat transplantation induces dermal adipose regeneration and reverses skin fibrosis through dedifferentiation and redifferentiation of adipocytes. Stem Cell Res Ther. 2022;13(1):499.
Cras A, Farge D, Carmoi T, et al. Update on mesenchymal stem cell-based therapy in lupus and scleroderma. Arthritis Res Ther. 2015;17:301.
Czirják L, Foeldvari I, Müller-Ladner U. Skin involvement in systemic sclerosis. Rheumatology (Oxford). 2008;47(5):v44–5.
Cui J, Jin L, Ding M, et al. Efficacy and safety of mesenchymal stem cells in the treatment of systemic sclerosis: a systematic review and meta-analysis. Stem Cell Res Ther. 2022;13(1):118.
Volarevic V, Markovic BS, Gazdic M, et al. Ethical and safety issues of stem cell-based therapy. Int J Med Sci. 2018;15(1):36–45.
Varderidou-Minasian S, Lorenowicz MJ. Mesenchymal stromal/stem cell-derived extracellular vesicles in tissue repair: challenges and opportunities. Theranostics. 2020;10(13):5979–97.
van Niel G, D’Angelo G, Raposo G. Shedding light on the cell biology of extracellular vesicles. Nat Rev Mol Cell Biol. 2018;19(4):213–28.
Keshtkar S, Azarpira N, Ghahremani MH. Mesenchymal stem cell-derived extracellular vesicles: novel frontiers in regenerative medicine. Stem Cell Res Ther. 2018;9(1):63.
Rozier P, Maumus M, Bony C, et al. Extracellular vesicles are more potent than adipose mesenchymal stromal cells to exert an anti-fibrotic effect in an in vitro model of systemic sclerosis. Int J Mol Sci. 2021;22(13):6834.
Phinney DG, Pittenger MF. Concise review: MSC-derived exosomes for cell-free therapy. Stem Cells. 2017;35(4):851–8.
Milbank E, Dragano NRV, González-García I, et al. Small extracellular vesicle-mediated targeting of hypothalamic AMPKα1 corrects obesity through BAT activation. Nat Metab. 2021;3(10):1415–31.
Zhuang WZ, Lin YH, Su LJ, et al. Mesenchymal stem/stromal cell-based therapy: mechanism, systemic safety and biodistribution for precision clinical applications. J Biomed Sci. 2021;28(1):28.
Herrmann IK, Wood MJA, Fuhrmann G. Extracellular vesicles as a next-generation drug delivery platform. Nat Nanotechnol. 2021;16(7):748–59.
Rockel JS, Rabani R, Viswanathan S. Anti-fibrotic mechanisms of exogenously-expanded mesenchymal stromal cells for fibrotic diseases. Semin Cell Dev Biol. 2020;101:87–103.
Rozier P, Maumus M, Maria ATJ, et al. Lung fibrosis Is improved by extracellular vesicles from IFNγ-primed mesenchymal stromal cells in murine systemic sclerosis. Cells. 2021;10(10):2727.
Rozier P, Maumus M, Maria ATJ, et al. Mesenchymal stromal cells-derived extracellular vesicles alleviate systemic sclerosis via miR-29a-3p. J Autoimmun. 2021;121:102660.
Baral H, Uchiyama A, Yokoyama Y, et al. Antifibrotic effects and mechanisms of mesenchymal stem cell-derived exosomes in a systemic sclerosis mouse model: possible contribution of miR-196b-5p. J Dermatol Sci. 2021;104(1):39–47.
Yu Y, Shen L, Xie X, et al. The therapeutic effects of exosomes derived from human umbilical cord mesenchymal stem cells on scleroderma. Tissue Eng Regen Med. 2022;19(1):141–50.
Guo L, Lai P, Wang Y, et al. Extracellular vesicles derived from mesenchymal stem cells prevent skin fibrosis in the cGVHD mouse model by suppressing the activation of macrophages and B cells immune response. Int Immunopharmacol. 2020;84:106541.
Hostettler KE, Gazdhar A, Khan P, et al. Multipotent mesenchymal stem cells in lung fibrosis. PLoS ONE. 2017;12(8):e0181946.
Toledo DM, Pioli PA. Macrophages in systemic sclerosis: novel insights and therapeutic implications. Curr Rheumatol Rep. 2019;21(7):31.
Sarvar DP, Effatpanah H, Akbarzadehlaleh P, et al. Mesenchymal stromal cell-derived extracellular vesicles: novel approach in hematopoietic stem cell transplantation. Stem Cell Res Ther. 2022;13(1):202.
Acknowledgements
The authors acknowledge the support from the National Natural Science Foundation of China (No. 81871277) and innovative research team of high-level local universities in Shanghai-Clinical and basic research on the prevention and treatment of some inflammatory diseases by integrative medicine.
Funding
This study was supported by the National Natural Science Foundation of China (No. 81871277) and innovative research team of high-level local universities in Shanghai-Clinical and basic research on the prevention and treatment of some inflammatory diseases by integrative medicine.
Author information
Authors and Affiliations
Contributions
These authors contributed equally: YX, ZH and YW. All authors read and approved the final version.
Corresponding authors
Ethics declarations
Competing interests
The authors declare that they have no competing interests.
Ethics approval and consent to participate
Not applicable.
Consent for publication
All of the authors approved the manuscript for publication.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Xiao, Y., Huang, Z., Wang, Y. et al. Progress in research on mesenchymal stem cells and their extracellular vesicles for treating fibrosis in systemic sclerosis. Clin Exp Med 23, 2997–3009 (2023). https://doi.org/10.1007/s10238-023-01136-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10238-023-01136-8