Abstract
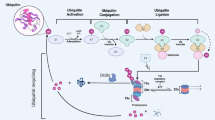
Reversible protein ubiquitination represents an essential determinator of cellular homeostasis, and the ubiquitin-specific enzymes, particularly deubiquitinases (DUBs), are emerging as promising targets for drug development. DUBs are composed of seven different subfamilies, out of which ubiquitin-specific proteases (USPs) are the largest family with 56 members. One of the well-characterized USPs is USP1, which contributes to several cellular biological processes including DNA damage response, immune regulation, cell proliferation, apoptosis, and migration. USP1 levels and activity are regulated by multiple mechanisms, including transcription regulation, phosphorylation, autocleavage, and proteasomal degradation, ensuring that the cellular function of USP1 is performed in a suitably modulated spatio-temporal manner. Moreover, USP1 with deregulated expression and activity are found in several human cancers, indicating that targeting USP1 is a feasible therapeutic approach in anti-cancer treatment. In this review, we highlight the essential role of USP1 in cancer development and the regulatory landscape of USP1 activity, which might provide novel insights into cancer treatment.
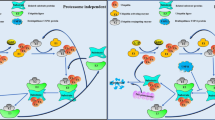
Graphical abstract








Similar content being viewed by others
Data availability
Not applicable.
References
Huibregtse JM. UPS shipping and handling. Cell. 2005;120(1):2–4. https://doi.org/10.1016/j.cell.2004.12.029.
Cruz Walma DA, Chen Z, Bullock AN, Yamada KM. Ubiquitin ligases: guardians of mammalian development. Nat Rev Mol Cell Biol. 2022;23(5):350–67. https://doi.org/10.1038/s41580-021-00448-5.
Rape M. Ubiquitylation at the crossroads of development and disease. Nat Rev Mol Cell Biol. 2018;19(1):59–70. https://doi.org/10.1038/nrm.2017.83.
Doerr A. Comprehensive mapping of ubiquitination. Nat Methods. 2018;15(9):651. https://doi.org/10.1038/s41592-018-0122-z.
Komander D, Rape M. The ubiquitin code. Annu Rev Biochem. 2012;81:203–29. https://doi.org/10.1146/annurev-biochem-060310-170328.
Komander D, Clague MJ, Urbé S. Breaking the chains: structure and function of the deubiquitinases. Nat Rev Mol Cell Biol. 2009;10(8):550–63. https://doi.org/10.1038/nrm2731.
Clague MJ, Urbé S, Komander D. Breaking the chains: deubiquitylating enzyme specificity begets function. Nat Rev Mol Cell Biol. 2019;20(6):338–52. https://doi.org/10.1038/s41580-019-0099-1.
Fujiwara T, Saito A, Suzuki M, et al. Identification and chromosomal assignment of USP1, a novel gene encoding a human ubiquitin-specific protease. Genomics. 1998;54(1):155–8. https://doi.org/10.1006/geno.1998.5554.
Jang SW, Kim JM. Mutation of aspartic acid 199 in USP1 disrupts its deubiquitinating activity and impairs DNA repair. FEBS Lett. 2021;595(15):1997–2006. https://doi.org/10.1002/1873-3468.14152.
Cohn MA, Kee Y, Haas W, Gygi SP, D’Andrea AD. UAF1 is a subunit of multiple deubiquitinating enzyme complexes. J Biol Chem. 2009;284(8):5343–51. https://doi.org/10.1074/jbc.M808430200.
Young MJ, Hsu KC, Lin TE, Chang WC, Hung JJ. The role of ubiquitin-specific peptidases in cancer progression. J Biomed Sci. 2019;26(1):42. https://doi.org/10.1186/s12929-019-0522-0.
Pal A, Young MA, Donato NJ. Emerging potential of therapeutic targeting of ubiquitin-specific proteases in the treatment of cancer. Can Res. 2014;74(18):4955–66. https://doi.org/10.1158/0008-5472.Can-14-1211.
Kang JA, Jeon YJ. Emerging roles of USP18: from biology to pathophysiology. Int J Molr Sci. 2020. https://doi.org/10.3390/ijms21186825.
Islam MT, Chen F, Chen H. The oncogenic role of ubiquitin specific peptidase (USP8) and its signaling pathways targeting for cancer therapeutics. Arch Biochem Biophys. 2021;701:108811. https://doi.org/10.1016/j.abb.2021.108811.
Wang F, Ning S, Yu B, Wang Y. USP14: structure, function, and target inhibition. Front Pharmacol. 2021;12:801328. https://doi.org/10.3389/fphar.2021.801328.
Li Q, Ye C, Tian T, et al. The emerging role of ubiquitin-specific protease 20 in tumorigenesis and cancer therapeutics. Cell Death Dis. 2022;13(5):434. https://doi.org/10.1038/s41419-022-04853-2.
Chauhan R, Bhat AA, Masoodi T, et al. Ubiquitin-specific peptidase 37: an important cog in the oncogenic machinery of cancerous cells. J Exp Clini Cancer Res CR. 2021;40(1):356. https://doi.org/10.1186/s13046-021-02163-7.
Morás AM, Henn JG, Steffens Reinhardt L, Lenz G, Moura DJ. Recent developments in drug delivery strategies for targeting DNA damage response in glioblastoma. Life Sci. 2021;287:120128. https://doi.org/10.1016/j.lfs.2021.120128.
Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–74. https://doi.org/10.1016/j.cell.2011.02.013.
O’Connor MJ. Targeting the DNA damage response in cancer. Mol Cell. 2015;60(4):547–60. https://doi.org/10.1016/j.molcel.2015.10.040.
Roos WP, Kaina B. DNA damage-induced cell death: from specific DNA lesions to the DNA damage response and apoptosis. Cancer Lett. 2013;332(2):237–48. https://doi.org/10.1016/j.canlet.2012.01.007.
Nalepa G, Clapp DW. Fanconi anaemia and cancer: an intricate relationship. Nat Rev Cancer. 2018;18(3):168–85. https://doi.org/10.1038/nrc.2017.116.
Sharp MF, Bythell-Douglas R, Deans AJ, Crismani W. The Fanconi anemia ubiquitin E3 ligase complex as an anti-cancer target. Mol Cell. 2021;81(11):2278–89. https://doi.org/10.1016/j.molcel.2021.04.023.
Semlow DR, Walter JC. Mechanisms of vertebrate DNA interstrand cross-link repair. Annu Rev Biochem. 2021;90:107–35. https://doi.org/10.1146/annurev-biochem-080320-112510.
Zhang Y, Zhou X, Huang P. Fanconi anemia and ubiquitination. J Genet Genom Yi Chuan Xue Bao. 2007;34(7):573–80. https://doi.org/10.1016/s1673-8527(07)60065-4.
Lemonidis K, Arkinson C, Rennie ML, Walden H. Mechanism, specificity, and function of FANCD2-FANCI ubiquitination and deubiquitination. FEBS J. 2021. https://doi.org/10.1111/febs.16077.
Kim JM, Parmar K, Huang M, et al. Inactivation of murine Usp1 results in genomic instability and a Fanconi anemia phenotype. Dev Cell. 2009;16(2):314–20. https://doi.org/10.1016/j.devcel.2009.01.001.
Oestergaard VH, Langevin F, Kuiken HJ, et al. Deubiquitination of FANCD2 is required for DNA crosslink repair. Mol Cell. 2007;28(5):798–809. https://doi.org/10.1016/j.molcel.2007.09.020.
Liang F, Miller AS, Longerich S, et al. DNA requirement in FANCD2 deubiquitination by USP1-UAF1-RAD51AP1 in the Fanconi anemia DNA damage response. Nat Commun. 2019;10(1):2849. https://doi.org/10.1038/s41467-019-10408-5.
Huang TT, Nijman SM, Mirchandani KD, et al. Regulation of monoubiquitinated PCNA by DUB autocleavage. Nat Cell Biol. 2006;8(4):339–47. https://doi.org/10.1038/ncb1378.
Hoege C, Pfander B, Moldovan GL, Pyrowolakis G, Jentsch S. RAD6-dependent DNA repair is linked to modification of PCNA by ubiquitin and SUMO. Nature. 2002;419(6903):135–41. https://doi.org/10.1038/nature00991.
Friedberg EC, Lehmann AR, Fuchs RP. Trading places: how do DNA polymerases switch during translesion DNA synthesis? Mol Cell. 2005;18(5):499–505. https://doi.org/10.1016/j.molcel.2005.03.032.
Lee KY, Yang K, Cohn MA, et al. Human ELG1 regulates the level of ubiquitinated proliferating cell nuclear antigen (PCNA) through Its interactions with PCNA and USP1. J Biol Chem. 2010;285(14):10362–9. https://doi.org/10.1074/jbc.M109.092544.
Jones MJ, Colnaghi L, Huang TT. Dysregulation of DNA polymerase κ recruitment to replication forks results in genomic instability. EMBO J. 2012;31(4):908–18. https://doi.org/10.1038/emboj.2011.457.
Lim KS, Li H, Roberts EA, et al. USP1 is required for replication fork protection in BRCA1-deficient tumors. Mol Cell. 2018;72(6):925-941.e924. https://doi.org/10.1016/j.molcel.2018.10.045.
Guervilly JH, Renaud E, Takata M, Rosselli F. USP1 deubiquitinase maintains phosphorylated CHK1 by limiting its DDB1-dependent degradation. Hum Mol Genet. 2011;20(11):2171–81. https://doi.org/10.1093/hmg/ddr103.
Yu Z, Song H, Jia M, et al. USP1-UAF1 deubiquitinase complex stabilizes TBK1 and enhances antiviral responses. J Exp Med. 2017;214(12):3553–63. https://doi.org/10.1084/jem.20170180.
Omilusik KD, Nadjsombati MS, Yoshida TM, et al. Ubiquitin specific protease 1 expression and function in T cell immunity. J Immunol (Baltimore Md: 1950). 2021;207(5):1377–87. https://doi.org/10.4049/jimmunol.2100303.
Song H, Zhao C, Yu Z, et al. UAF1 deubiquitinase complexes facilitate NLRP3 inflammasome activation by promoting NLRP3 expression. Nat Commun. 2020;11(1):6042. https://doi.org/10.1038/s41467-020-19939-8.
Knochelmann HM, Dwyer CJ, Bailey SR, et al. When worlds collide: Th17 and Treg cells in cancer and autoimmunity. Cell Mol Immunol. 2018;15(5):458–69. https://doi.org/10.1038/s41423-018-0004-4.
Reiner SL. Development in motion: helper T cells at work. Cell. 2007;129(1):33–6. https://doi.org/10.1016/j.cell.2007.03.019.
Sfanos KS, Bruno TC, Maris CH, et al. Phenotypic analysis of prostate-infiltrating lymphocytes reveals TH17 and Treg skewing. Clini Cancer Res Off J Am Assoc Cancer Res. 2008;14(11):3254–61. https://doi.org/10.1158/1078-0432.Ccr-07-5164.
Duan MC, Han W, Jin PW, et al. Disturbed Th17/Treg balance in patients with non-small cell lung cancer. Inflammation. 2015;38(6):2156–65. https://doi.org/10.1007/s10753-015-0198-x.
Littman DR, Rudensky AY. Th17 and regulatory T cells in mediating and restraining inflammation. Cell. 2010;140(6):845–58. https://doi.org/10.1016/j.cell.2010.02.021.
Geng J, Yu S, Zhao H, et al. The transcriptional coactivator TAZ regulates reciprocal differentiation of T(H)17 cells and T(reg) cells. Nat Immunol. 2017;18(7):800–12. https://doi.org/10.1038/ni.3748.
Zhu X, Wang P, Zhan X, et al. USP1-regulated reciprocal differentiation of Th17 cells and Treg cells by deubiquitinating and stabilizing TAZ. Cell Mol Immunol. 2023;20(3):252–63. https://doi.org/10.1038/s41423-022-00969-9.
Tosolini M, Kirilovsky A, Mlecnik B, et al. Clinical impact of different classes of infiltrating T cytotoxic and helper cells (Th1, th2, treg, th17) in patients with colorectal cancer. Can Res. 2011;71(4):1263–71. https://doi.org/10.1158/0008-5472.Can-10-2907.
Martin-Orozco N, Chung Y, Chang SH, Wang YH, Dong C. Th17 cells promote pancreatic inflammation but only induce diabetes efficiently in lymphopenic hosts after conversion into Th1 cells. Eur J Immunol. 2009;39(1):216–24. https://doi.org/10.1002/eji.200838475.
Lasorella A, Stegmüller J, Guardavaccaro D, et al. Degradation of Id2 by the anaphase-promoting complex couples cell cycle exit and axonal growth. Nature. 2006;442(7101):471–4. https://doi.org/10.1038/nature04895.
Williams SA, Maecker HL, French DM, et al. USP1 deubiquitinates ID proteins to preserve a mesenchymal stem cell program in osteosarcoma. Cell. 2011;146(6):918–30. https://doi.org/10.1016/j.cell.2011.07.040.
Lee JK, Chang N, Yoon Y, et al. USP1 targeting impedes GBM growth by inhibiting stem cell maintenance and radioresistance. Neuro Oncol. 2016;18(1):37–47. https://doi.org/10.1093/neuonc/nov091.
Das DS, Das A, Ray A, et al. Blockade of deubiquitylating enzyme USP1 inhibits DNA repair and triggers apoptosis in multiple myeloma cells. Clini Cancer Res Off J Am Assoc Cancer Res. 2017;23(15):4280–9. https://doi.org/10.1158/1078-0432.Ccr-16-2692.
Meng D, Li D. Ubiquitin-specific protease 1 overexpression indicates poor prognosis and promotes proliferation, migration, and invasion of gastric cancer cells. Tissue cell. 2022;74:101723. https://doi.org/10.1016/j.tice.2021.101723.
Ma A, Tang M, Zhang L, et al. USP1 inhibition destabilizes KPNA2 and suppresses breast cancer metastasis. Oncogene. 2019;38(13):2405–19. https://doi.org/10.1038/s41388-018-0590-8.
Wu Y, Wang Y, Lin Y, et al. Dub3 inhibition suppresses breast cancer invasion and metastasis by promoting Snail1 degradation. Nat Commun. 2017;8:14228. https://doi.org/10.1038/ncomms14228.
Jing C, Li X, Zhou M, et al. The PSMD14 inhibitor Thiolutin as a novel therapeutic approach for esophageal squamous cell carcinoma through facilitating SNAIL degradation. Theranostics. 2021;11(12):5847–62. https://doi.org/10.7150/thno.46109.
Guo X, Zhu R, Luo A, et al. EIF3H promotes aggressiveness of esophageal squamous cell carcinoma by modulating snail stability. J Exp Clini Cancer Res CR. 2020;39(1):175. https://doi.org/10.1186/s13046-020-01678-9.
Liu T, Yu J, Deng M, et al. CDK4/6-dependent activation of DUB3 regulates cancer metastasis through SNAIL1. Nat Commun. 2017;8:13923. https://doi.org/10.1038/ncomms13923.
Sonego M, Pellarin I, Costa A, et al. USP1 links platinum resistance to cancer cell dissemination by regulating snail stability. Sci Adv. 2019;5(5):eaav3235. https://doi.org/10.1126/sciadv.aav3235.
Kamata YU, Sumida T, Kobayashi Y, et al. Introduction of ID2 enhances invasiveness in ID2-null oral squamous cell carcinoma cells via the SNAIL axis. Cancer Genom Proteom. 2016;13(6):493–7. https://doi.org/10.21873/cgp.20012.
Li N, Wu L, Zuo X, et al. USP1 Promotes GC metastasis via stabilizing ID2. Dis Mark. 2021;2021:3771990. https://doi.org/10.1155/2021/3771990.
Liao Y, Shao Z, Liu Y, et al. USP1-dependent RPS16 protein stability drives growth and metastasis of human hepatocellular carcinoma cells. J Exp Clini Cancer Res CR. 2021;40(1):201. https://doi.org/10.1186/s13046-021-02008-3.
Han D, Wang L, Chen B, et al. USP1-WDR48 deubiquitinase complex enhances TGF-β induced epithelial-mesenchymal transition of TNBC cells via stabilizing TAK1. Cell Cycle (Georgetown, Tex). 2021;20(3):320–31. https://doi.org/10.1080/15384101.2021.1874695.
Mussell A, Shen H, Chen Y, et al. USP1 regulates TAZ protein stability through ubiquitin modifications in breast cancer. Cancers. 2020. https://doi.org/10.3390/cancers12113090.
Yuan P, Feng Z, Huang H, et al. USP1 inhibition suppresses the progression of osteosarcoma via destabilizing TAZ. Int J Biol Sci. 2022;18(8):3122–36. https://doi.org/10.7150/ijbs.65428.
Ohsugi T, Yamaguchi K, Zhu C, et al. Anti-apoptotic effect by the suppression of IRF1 as a downstream of Wnt/β-catenin signaling in colorectal cancer cells. Oncogene. 2019;38(32):6051–64. https://doi.org/10.1038/s41388-019-0856-9.
Liao Y, Liu Y, Shao Z, et al. A new role of GRP75-USP1-SIX1 protein complex in driving prostate cancer progression and castration resistance. Oncogene. 2021;40(25):4291–306. https://doi.org/10.1038/s41388-021-01851-0.
Liao Y, Sun W, Shao Z, et al. A SIX1 degradation inducer blocks excessive proliferation of prostate cancer. Int J Biol Sci. 2022;18(6):2439–51. https://doi.org/10.7150/ijbs.67873.
Yu Y, Davicioni E, Triche TJ, Merlino G. The homeoprotein six1 transcriptionally activates multiple protumorigenic genes but requires ezrin to promote metastasis. Can Res. 2006;66(4):1982–9. https://doi.org/10.1158/0008-5472.Can-05-2360.
Li Z, Tian T, Lv F, et al. Six1 promotes proliferation of pancreatic cancer cells via upregulation of cyclin D1 expression. PloS one. 2013;8(3):e59203. https://doi.org/10.1371/journal.pone.0059203.
Antonenko SV, Gurianov DS, Telegeev GD. Colocalization of USP1 and PH domain of BcrAbl oncoprotein in terms of cronic myeloid leukemia cell rearrangements. Tsitol Genet. 2016;50(4):11–5.
Mistry H, Hsieh G, Buhrlage SJ, et al. Small-molecule inhibitors of USP1 target ID1 degradation in leukemic cells. Mol Cancer Ther. 2013;12(12):2651–62. https://doi.org/10.1158/1535-7163.Mct-13-0103-t.
Jiang S, Wang X, He Y, et al. Suppression of USP7 induces BCR-ABL degradation and chronic myelogenous leukemia cell apoptosis. Cell Death Dis. 2021;12(5):456. https://doi.org/10.1038/s41419-021-03732-6.
Kuang X, Xiong J, Lu T, et al. Inhibition of USP1 induces apoptosis via ID1/AKT pathway in B-cell acute lymphoblastic leukemia cells. Int J Med Sci. 2021;18(1):245–55. https://doi.org/10.7150/ijms.47597.
Nijman SM, Huang TT, Dirac AM, et al. The deubiquitinating enzyme USP1 regulates the Fanconi anemia pathway. Mol Cell. 2005;17(3):331–9. https://doi.org/10.1016/j.molcel.2005.01.008.
Rego MA, Harney JA, Mauro M, Shen M, Howlett NG. Regulation of the activation of the Fanconi anemia pathway by the p21 cyclin-dependent kinase inhibitor. Oncogene. 2012;31(3):366–75. https://doi.org/10.1038/onc.2011.237.
Rahme GJ, Zhang Z, Young AL, et al. PDGF engages an E2f-USP1 signaling pathway to support id2-mediated survival of proneural glioma Cells. Can Res. 2016;76(10):2964–76. https://doi.org/10.1158/0008-5472.Can-15-2157.
Ma L, Lin K, Chang G, et al. Aberrant activation of β-catenin signaling drives glioma tumorigenesis via USP1-mediated stabilization of EZH2. Can Res. 2019;79(1):72–85. https://doi.org/10.1158/0008-5472.Can-18-1304.
Mohanty A, Nam A, Pozhitkov A, et al. A non-genetic mechanism involving the integrin β4/paxillin axis contributes to chemoresistance in lung cancer. iScience. 2020;23(9):101496. https://doi.org/10.1016/j.isci.2020.101496.
Gong H, Liu L, Cui L, Ma H, Shen L. ALKBH5-mediated m6A-demethylation of USP1 regulated T-cell acute lymphoblastic leukemia cell glucocorticoid resistance by Aurora B. Mol Carcinog. 2021;60(9):644–57. https://doi.org/10.1002/mc.23330.
Yates LA, Norbury CJ, Gilbert RJ. The long and short of microRNA. Cell. 2013;153(3):516–9. https://doi.org/10.1016/j.cell.2013.04.003.
Krol J, Loedige I, Filipowicz W. The widespread regulation of microRNA biogenesis, function and decay. Nat Rev Genet. 2010;11(9):597–610. https://doi.org/10.1038/nrg2843.
Dong H, Lei J, Ding L, et al. MicroRNA: function, detection, and bioanalysis. Chem Rev. 2013;113(8):6207–33. https://doi.org/10.1021/cr300362f.
Flammang I, Reese M, Yang Z, Eble JA, Dhayat SA. Tumor-suppressive miR-192–5p has prognostic value in pancreatic ductal adenocarcinoma. Cancers. 2020. https://doi.org/10.3390/cancers12061693.
Yin S, Jin W, Qiu Y, et al. Solamargine induces hepatocellular carcinoma cell apoptosis and autophagy via inhibiting LIF/miR-192-5p/CYR61/Akt signaling pathways and eliciting immunostimulatory tumor microenvironment. J Hematol Oncol. 2022;15(1):32. https://doi.org/10.1186/s13045-022-01248-w.
Zou P, Zhu M, Lian C, et al. miR-192-5p suppresses the progression of lung cancer bone metastasis by targeting TRIM44. Sci Rep. 2019;9(1):19619. https://doi.org/10.1038/s41598-019-56018-5.
Zhou S, Xiong M, Dai G, et al. MicroRNA-192-5p suppresses the initiation and progression of osteosarcoma by targeting USP1. Oncol Lett. 2018;15(5):6947–56. https://doi.org/10.3892/ol.2018.8180.
Xu J, Li B, Song W, et al. Tumor suppressor functions of miRNA-375 in nasopharyngeal carcinoma through inhibition of ubiquitin-specific protease 1 expression. Int J Biochem Cell Biol. 2021;141:106092. https://doi.org/10.1016/j.biocel.2021.106092.
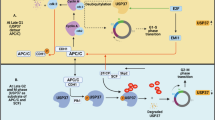
Cotto-Rios XM, Jones MJ, Busino L, Pagano M, Huang TT. APC/CCdh1-dependent proteolysis of USP1 regulates the response to UV-mediated DNA damage. J Cell Biol. 2011;194(2):177–86. https://doi.org/10.1083/jcb.201101062.
Cataldo F, Peche LY, Klaric E, et al. CAPNS1 regulates USP1 stability and maintenance of genome integrity. Mol Cell Biol. 2013;33(12):2485–96. https://doi.org/10.1128/mcb.01406-12.
Cotto-Rios XM, Jones MJ, Huang TT. Insights into phosphorylation-dependent mechanisms regulating USP1 protein stability during the cell cycle. Cell Cycle (Georgetown, Tex). 2011;10(23):4009–16. https://doi.org/10.4161/cc.10.23.18501.
Villamil MA, Liang Q, Chen J, et al. Serine phosphorylation is critical for the activation of ubiquitin-specific protease 1 and its interaction with WD40-repeat protein UAF1. Biochemistry. 2012;51(45):9112–23. https://doi.org/10.1021/bi300845s.
Liu J, Zhu H, Zhong N, et al. Gene silencing of USP1 by lentivirus effectively inhibits proliferation and invasion of human osteosarcoma cells. Int J Oncol. 2016;49(6):2549–57. https://doi.org/10.3892/ijo.2016.3752.
Gonzalez-Fierro A, Dueñas-González A. Drug repurposing for cancer therapy, easier said than done. Semin Cancer Biol. 2021;68:123–31. https://doi.org/10.1016/j.semcancer.2019.12.012.
Pilié PG, Tang C, Mills GB, Yap TA. State-of-the-art strategies for targeting the DNA damage response in cancer. Nat Rev Clin Oncol. 2019;16(2):81–104. https://doi.org/10.1038/s41571-018-0114-z.
Chen J, Dexheimer TS, Ai Y, et al. Selective and cell-active inhibitors of the USP1/ UAF1 deubiquitinase complex reverse cisplatin resistance in non-small cell lung cancer cells. Chem Biol. 2011;18(11):1390–400. https://doi.org/10.1016/j.chembiol.2011.08.014.
Sourisseau T, Helissey C, Lefebvre C, et al. Translational regulation of the mRNA encoding the ubiquitin peptidase USP1 involved in the DNA damage response as a determinant of Cisplatin resistance. Cell Cycle (Georgetown, Tex). 2016;15(2):295–302. https://doi.org/10.1080/15384101.2015.1120918.
Cui SZ, Lei ZY, Guan TP, et al. Targeting USP1-dependent KDM4A protein stability as a potential prostate cancer therapy. Cancer Sci. 2020;111(5):1567–81. https://doi.org/10.1111/cas.14375.
Wang L, Hu T, Shen Z, et al. Inhibition of USP1 activates ER stress through Ubi-protein aggregation to induce autophagy and apoptosis in HCC. Cell Death Dis. 2022;13(11):951. https://doi.org/10.1038/s41419-022-05341-3.
Liu Y, Kong WY, Yu CF, et al. SNS-023 sensitizes hepatocellular carcinoma to sorafenib by inducing degradation of cancer drivers SIX1 and RPS16. Acta Pharmacol Sin. 2022. https://doi.org/10.1038/s41401-022-01003-4.
Chen Z, Ma Y, Guo Z, et al. Ubiquitin-specific protease 1 acts as an oncogene and promotes lenvatinib efficacy in hepatocellular carcinoma by stabilizing c-kit. Ann Hepatol. 2022;27(2):100669. https://doi.org/10.1016/j.aohep.2022.100669.
Liang Q, Dexheimer TS, Zhang P, et al. A selective USP1-UAF1 inhibitor links deubiquitination to DNA damage responses. Nat Chem Biol. 2014;10(4):298–304. https://doi.org/10.1038/nchembio.1455.
Dexheimer TS, Rosenthal AS, Luci DK, et al. Synthesis and structure-activity relationship studies of N-benzyl-2-phenylpyrimidin-4-amine derivatives as potent USP1/UAF1 deubiquitinase inhibitors with anticancer activity against nonsmall cell lung cancer. J Med Chem. 2014;57(19):8099–110. https://doi.org/10.1021/jm5010495.
Dexheimer TS, Rosenthal AS, Liang Q, et al. Discovery of ML323 as a novel inhibitor of the USP1/UAF1 deubiquitinase complex. In: Bethesda A, editor., et al., Probe reports from the NIH molecular libraries program. Bethesda: National Center for Biotechnology Information; 2010.
Lackner LL, Nunnari J. Small molecule inhibitors of mitochondrial division: tools that translate basic biological research into medicine. Chem Biol. 2010;17(6):578–83. https://doi.org/10.1016/j.chembiol.2010.05.016.
Dong L, He J, Luo L, Wang K. Targeting the interplay of autophagy and ROS for cancer therapy: an updated overview on phytochemicals. Pharmaceuticals (Basel, Switzerland). 2023. https://doi.org/10.3390/ph16010092.
Sun Y, Sha B, Huang W, et al. ML323, a USP1 inhibitor triggers cell cycle arrest, apoptosis and autophagy in esophageal squamous cell carcinoma cells. Apoptosis Int J Program cell Death. 2022;27(7–8):545–60. https://doi.org/10.1007/s10495-022-01736-x.
Lu Z, Zhang Z, Yang M, Xiao M. Ubiquitin-specific protease 1 inhibition sensitizes hepatocellular carcinoma cells to doxorubicin by ubiquitinated proliferating cell nuclear antigen-mediated attenuation of stemness. Anticancer Drugs. 2022;33(7):622–31. https://doi.org/10.1097/cad.0000000000001311.
Sowa ME, Bennett EJ, Gygi SP, Harper JW. Defining the human deubiquitinating enzyme interaction landscape. Cell. 2009;138(2):389–403. https://doi.org/10.1016/j.cell.2009.04.042.
Doherty LM, Mills CE, Boswell SA, et al. Integrating multi-omics data reveals function and therapeutic potential of deubiquitinating enzymes. eLife. 2022. https://doi.org/10.7554/eLife.72879.
Acknowledgements
We would like to thank Professor Qiu Li for the writing assistance and proofreading the article.
Funding
This research was supported by Science & Technology Department of Sichuan Province Funding Project (2022YFSY0027-Q.L) and Science & Technology Department of Sichuan Province Funding Project (2022NSFSC1348-PF.Z).
Author information
Authors and Affiliations
Contributions
PH and YHW conceptualized the study, contributed to the preparation of the initial draft of the manuscript, and designed the figures, and tables. PFZ conceptualized the study, critically revised the manuscript, and administered the project. QL supervised the project and contributed to manuscript revision. All authors have read and approved the submitted version.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests to declare.
Ethical approval and consent to participate
Not applicable.
Consent for publication
All authors have agreed to publish this manuscript.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Huang, P., Wang, Y., Zhang, P. et al. Ubiquitin-specific peptidase 1: assessing its role in cancer therapy. Clin Exp Med 23, 2953–2966 (2023). https://doi.org/10.1007/s10238-023-01075-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10238-023-01075-4